Blood pressure medications recalled after oxycodone found inside, the FDA warns
Taking an amazing can have serious health implications.
Although there are several lifestyle changes You can do to resolve high blood pressure, many Americans also take medication to help keep the levels at a healthy pace. Medicines can help Heart failure, renal failure, heart attack or stroke, according to the Cleveland Clinic, important considerations if you are one of the 116 million people affected by high blood pressure. But although these drugs are intended to improve your health, a variety is now subject to recall because of the presence of narcotic oxycodone. Read the rest to find out what you need to do if you have these blood pressure drugs at home.
In relation: Heart medication recalled after a dangerous mixture of labels, the FDA warns .
A wandering oxycodone tablet was found after a batch of blood pressure tablets was wrapped.
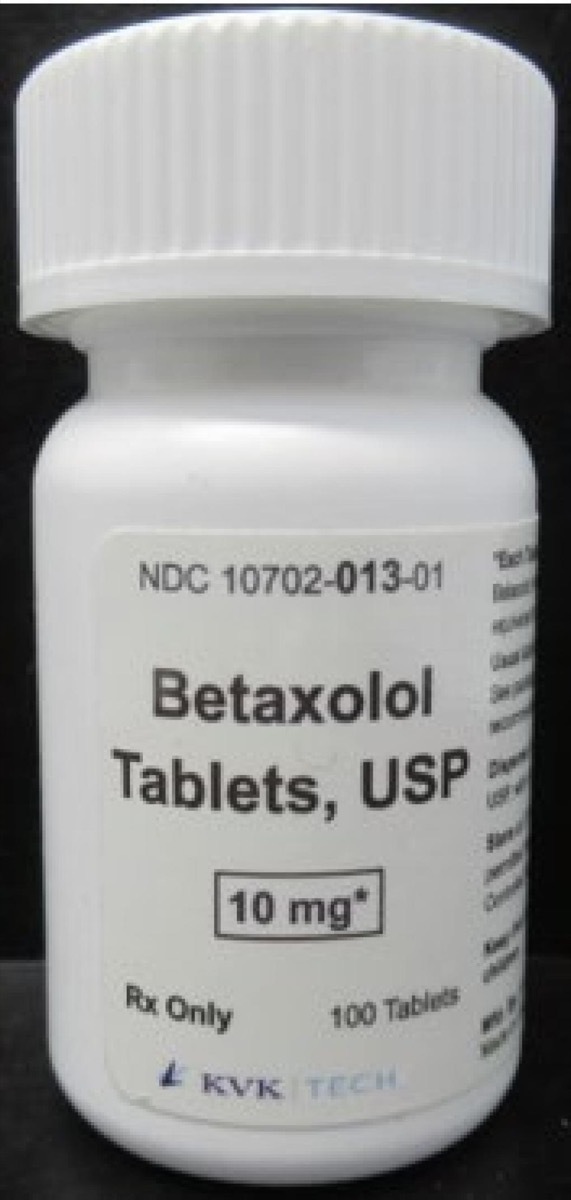
According to a October 3 reminder From the US Food and Drug Administration (FDA), KVK-Tech, Inc. voluntarily remembers a single batch of tablets of 10 milligrams of Betaxolol.
Depending on the review, the 10 mg of Betaxolol, round and film -coated pills, with a side displaying a "K" and the number "13." The pills were packed in white plastic bottles, each filled with 100 tablets, and distributed to wholesalers and retailers in the country. The batch bottles affected (lot number: 17853a) have an expiration date of June 2027.
The lot was recalled "as a precaution", due to the presence of an oxycodone hydrochloride - an amazing (also called opioid) which is a " popular abuse drug “Per la U.S. Drug Enforcement Administration (DEA).
According to the press release, the company has found a single tablet of oxycodone hydrochloride of five milligrams on the packaging line during line clearance - the process of ensuring that the equipment is free from materials - after the share of Betaxolol recalled.
In relation: 2 drugs recalled after a major mix: "serious adverse events", warns the FDA .
Taking an amazing can present a "serious health risk".

If more oxycodone pills were wrapped in error with or instead of betaxolol, it has serious health risks for different groups of patients, according to the FDA.
"The insertion of the Betaxolol package warns against the slowdown in heart rate in elderly patients, which is likely to be exacerbated by the administration of inadvertent opioids," said the FDA press release. "In addition, some patients have prescribed low -dose betaxolol may have compromised the cardiac and pulmonary function which is also likely to be exacerbated by an opioid."
In addition, people with opioid consumption disorders (oud), people at risk of oud, infants, children and the elderly can be negatively affected if they wrongly take a narcotic. This is particularly true "if a substantial number of oxycodone tablets have been introduced into a labeled bottle like Betaxolol," said the agency.
"Consequently, accidental exposure to a controlled substance, such as oxycodone, in this population of patients, is likely to slow down breathing, known as respiratory depression, which represents a serious health risk", said the press release.
In relation: Probiotics sold at Walmart and Amazon remembered for a "possible risk for health", warns the FDA .
Stop taking these pills if you have them.
The FDA notes that the tablets of betaxolol and oxycodone hydrochloride are similar, and there are "minor differences of appearance between the 10 mg of betaxolol and the oxycodone 5 mg" tablets.
In fact, even if you take tablets of 10 milligrams Betaxolol every day, you would probably not notice the difference, according to the FDA. AE0FCC31AE342FD3A1346EBB1F342FCB
Although KVK has received no reports from foreign tablets in Betaxolol tablet bottles, if you have received pills recalled, stop using them and send them immediately to KVK-Tech. The company will reimburse you the cost of buying the drug, the press release said.
If you encounter problems related to these tablets, contact your doctor or health care provider, according to the FDA.
In relation: Costco recalls the musk squash due to the risk of E. Coli, warns the FDA .
KVK actively informs customers.

KVK informed distributors and recall customers by e-mail and FEDEX Night Mail on September 26, said the FDA press release. The company is currently organizing the return of batch products recalled. The company also noted that a small number of bottles may have gone to retail pharmacies.
For questions about the recall, you can call KVK at 215-579-1842 Ext: 6002, Monday to Friday between 8 a.m. and 6 p.m. EASTERN Standard Time (East), or E-mail [Protected by e-mail] .
In relation: For more information, register for our daily newsletter .

5 red flags on emojis that your partner sends SMS, according to therapists

