4 drugs doctors never prescribe again
These drugs have been removed from the market for a good reason.

When you contest your health to a doctor, you might think that you are both the only parts to make crucial calls on your well-being. But the second when your doctor comes out a prescription cushion, this circle of trust is developing to include pharmaceutical companies and regulatory agencies. Unfortunately, as we all knowHigh -level reminders In which drugs are removed from the market, the pharmaceutical industry sometimes makes mistakes. Although all drugs include risks and advantages, some products have just been too dangerous to take, causing an avoidable - and irreversible - or even death assessment.
Meanwhile, many drugs with serious side effects remain on the market or come back after aShort reminder. It takes a long time for drugs to be taken from the permanence of the shelves, but this happens. Read the rest to find out more about four drugs than doctors will probably never prescribe and to hear the surprising stories of the reason why they are no longer prescribed.
Read this then:Large pharmacies block this common daily drug.
1 Vioxx for arthritis

Vioxx is a Cox-2 inhibitor which was once commonly used to treat arthritis. But in 2004, the drug manufacturer, Merck & Co., had removed the market when the news announced that it had been linked to 88,000 heart attacks between 1999 and 2003-38,000. At a time when it was removed from the market, it was estimated that more than 20 million patients had taken the medication. In 2007, Merck reached aRules of $ 4.85 billion To resolve thousands of proceedings, the biggest drug regulations in history, according to NPR.
"There is certainly a need for COX-2 inhibitors, but in the end, they were badly marketed by pharmaceutical companies to doctors to use higher doses," saidWilliam Soliman, PhD, BCMAS, founder and CEO ofAccreditation advice for medical affairs (ACMA) and a pharmaceutical framework that previously worked with Merck. "In my opinion, this has led to part of the cardiovascular risk," explains SolimanBetter life.
2 Bextra for pain relief

Valdecoxib, marked like Bextra, is a non-steroidal (NSAID) anti-inflammatory drug that wasOnce prescribed as analgesic. However, in 2005, the researchers learned that the drug caused serious and sometimes fatal cardiovascular complications such as the heart attack and stroke. Rarely, Bextra has also proven to cause two potentially fatal skin reactions called Stevens-Johnson syndrome and toxic epidermal necrolysis.
Having determined that Bextra had no particular advantage over similar pain relievers which did not cause such serious complications, theThe FDA has moved to interrupt its use.
3 Belviq for weight loss

Lorcaserin, marked like Belviq or Belviq XR, was previously prescribed as a weight loss drug after being approved in 2012. However, in 2020, the FDA asked doctors to stop prescribing Lorcaserin after a study revealed a link between medication and cancer.
"We take this action because we think theLorcaserin's risks prevail over its advantages Based on our examination completed the results of a randomized clinical trial evaluating security, "wrote the FDA in its recall ad." Health professionals should stop prescribing and providing Lorcaserin to patients. Contact patients who are currently taking Lorcaserine, inform them of the increased occurrence of cancer observed in the clinical trial and ask them to stop taking the medication. Discuss drugs or alternative weight loss strategies with your patients, "said the regulatory body.AE0FCC31AE342FD3A1346EBB1F342FCB
For more health information sent directly to your reception box,Register for our daily newsletter.
4 Raptiva for psoriasis
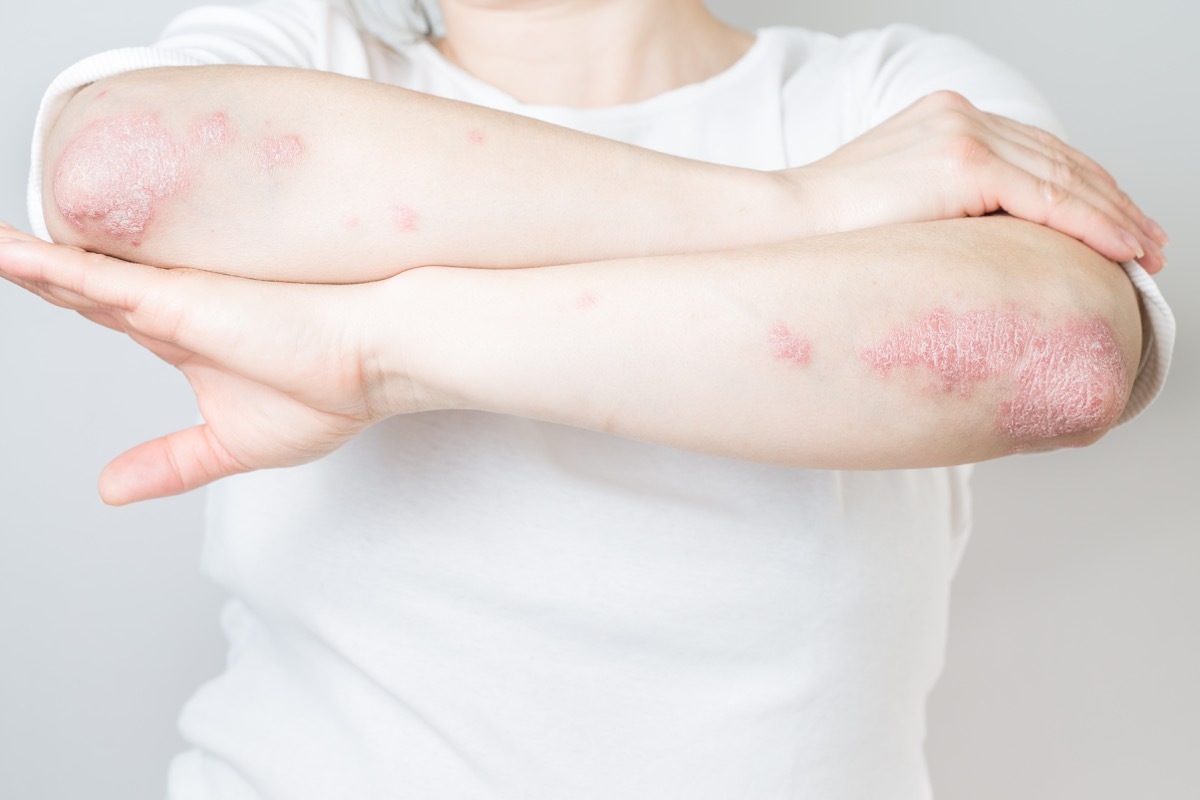
Efalizumab, marked like Raptiva, was an injectable medication once a week used to treat adults with moderate to severe plaque psoriasis. However, in 2009, its producer launched "A voluntary and progressive withdrawal of the product of the American market. ""
Indeed, scientists have learned that the drug had been linked to the progressive multifocal leukoencephalopathy (PML), a rare but serious neurological disease caused by a virus that affects the central nervous system. In its opinion of drug withdrawal, the FDA noted that there is "no effective treatment known for the LMP", and although it is unlikely for anyone taking Raptiva to develop the disease, it can ultimately be fatal in those who contract it.
Best Life offers the most recent information from high -level experts, new research and health agencies, but our content is not supposed to replace professional advice. Regarding the medication you take or any other health issue you have, always consult your health care provider directly.

Coronavirus kills people with this common condition in a week

