These tests could have "false results", declares FDA in a new warning
You may want to reconsider the use of this blood test.
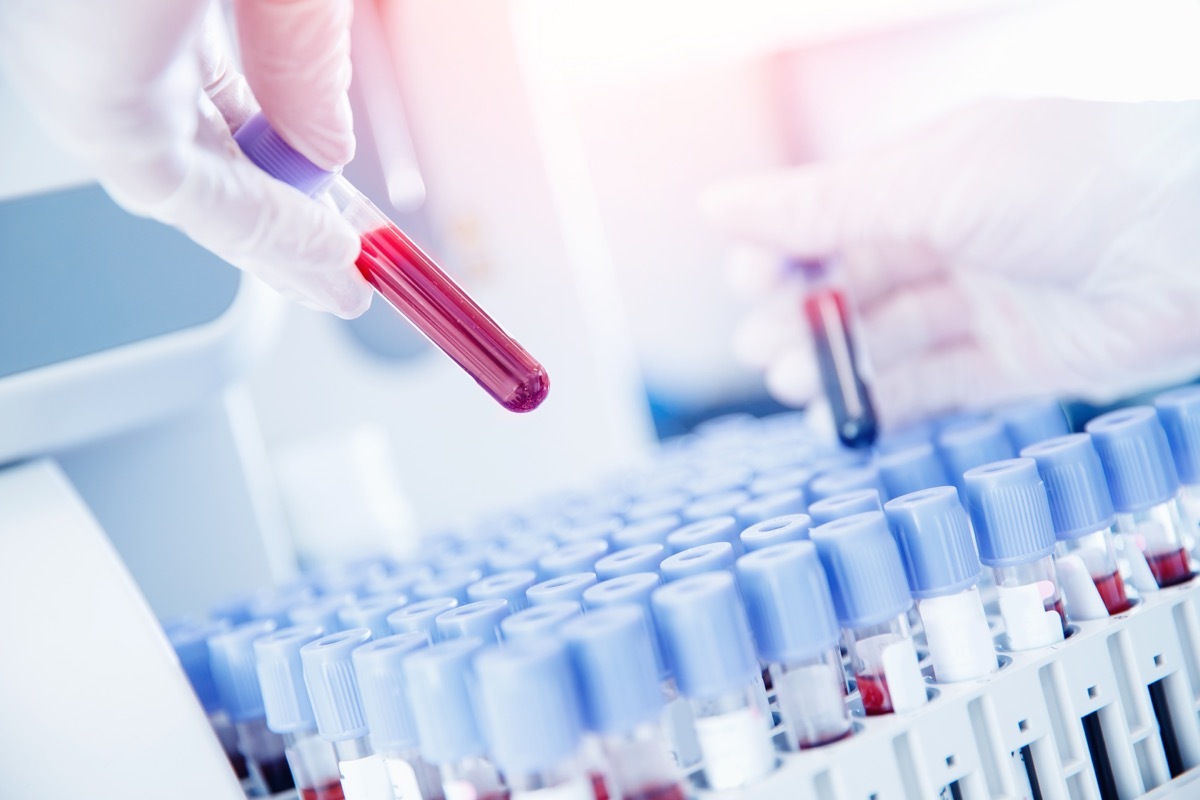
We all strivekeep our health And general well-being, many of us make healthy choices, heading to the gym several times a week and checking with our doctors every year. One of the ways of health care providers retains tabs on our health consists of crossing blood tests - and all that is not the most enjoyable part of a balance sheet, it's essential. These tests can not only checkDifferent diseases And conditions, but they can also make sure that your organs work properly and determine whether the treatments are effective, the national heart, the lung and the bloodstream of blood. However, these tests are not always what they seem, as well as the Food and Drug Administration (FDA) have just warned a test of a particular test. Read more about the blood test you might want to reconsider.
RELATED:If you use this common medicine, the FDA has a new major warning for you.
Blood tests have several uses, but some approaches have aroused debate and controversy.

Blood tests play a central role in maintaining your overall health and can sometimes alert us to conditions that we did not even know that we had. In fact, actorBen Stiller makes titles after learning that he hadProstate cancer Thanks to a prostate-specific antigen test (PSA). Stiller called the "controversial" test, as its use is debated between health professionals.
And this is not the only test that has been a hot topic of discussion. Hulu fansThe losswill recognize the name Theranos, a company that claimed to be able to perform hundreds of tests with just aunique blood drop. Led by his CEO now infamenElizabeth HolmesThe Company's claims ended up being false and theanos was closed among fraud allegations. Now, additional marketed blood tests have increased in popularity, but there are persistent reliability issues.
RELATED:The CDC and the FDA has just published a warning on this type of marijuana.
Some types of blood tests could actually produce "false results".

When it comes to keeping children safe, parents are committed to making an extra mile. The parents waiting, however, will want to be cautious when usingNon invasive prenatal screening Tests (NIPs), which are also called DNA tests without non-invasive prenatal tests (NIPT), according to a warning issued by the FDA on April 19th.AE0FCC31AE342FD3A1346EBB1F342FCB
These tests analyze a blood sample of a pregnant person, looking for "signs of genetic abnormalities in a fetus," said the agency. But NIPS tests are screening tests rather than diagnostic tests, which means that they provide only information about therisk a genetic anomaly. To confirm whether these results are accurate and the fetus has this anomaly, additional tests may be necessary.
The FDA issued the warning due to widely used tests.

As reported on the edge, these tests are considered "Tests developed by the laboratory, "which are not subject to consideration by the FDA before being sold and announced by companies. Parents might not be aware of the limitations of these tests and that many requests for confirmation of their baby will be born happy and in Good health, the use of NIPs The tests have increased late, the FDA said.
"While non-invasive genetic prenatal screening tests are widely used today, these tests have not been examined by the FDA and can make claims on their performance and use that are not based on sound science" , "Jeff Shuren, MD, JD, Director of the FDA Cameras and Radiological Health, said in the warning.
RELATED:For more information up to date, sign up for our daily newsletter.
If you are going to use a NIP or NIPT test, the FDA advises you first.

These tests claim to provide a "peace of mind", but they are particularly limited when screening the rarer conditions, which often leads to a false positive (which means that the fetus is not really affected), said the FDA. The tests can also cause confusion for parents by precisely detecting a chromosomal abnormality, which can only be present in the placenta and not in the fetus. Earlier this year,The New York Times watched in these prenatal blood tests, noting that whenRare disorders test, like CRI-DU-CAT syndrome and Wolf-Hirschhorn syndrome, the results were incorrect of 80% or more time.
The FDA stated that it is aware of these media reports and "critical health decisions" because of these test results. "Without proper understanding of how these tests should be used, people can make inappropriate health care decisions about their pregnancy," said Shuren. "We urge patients to discuss the benefits and risks of these tests with a genetic advisor or other health care provider before making decisions based on the results of these tests."
The FDA plans to take additional steps on these tests.

These tests are considered as medical devices under the Federal Food, Drugs and Cosmetics Act, and the policy of "discretion of implementation" has not changed since 1976 when changes in medical devices have been added. However, the Agency seeks to "establish a modern regulatory framework" for all tests, depending on the declaration of warning. The Agency also noted that it will continue to monitor these security issues, as well as the use of NIPS tests.
RELATED:The "crazy" Mark Ruffalo discovered that he had a brain tumor.
Becoming a covid could age your 5 -year -old body, shocking research discoveries

Never use dryer leaves with these items, experts warn
