If you take this medicine, stop now, says FDA
The effectiveness of the medication can be compromised, putting those who use it at risk of serious prejudice.
Whether you keep your antibiotics away from your insulin light or tobacco sources in the refrigerator, many people are intelled how important it is to ensure that their life maintenance medications are properly stored. Unfortunately, the Food & Drug Administration (FDA) now advises individuals who have been prescribed a particular drug to stop taking it after discovering that inappropriate storage conditions may mean that this poses a serious risk for users. Continue to discover if you should get rid of this medicine and call your doctor now.
RELATED:If you bought these supplements, stop using them immediately, says FDA.
Two atovaquone oral suspension lots were recalled.
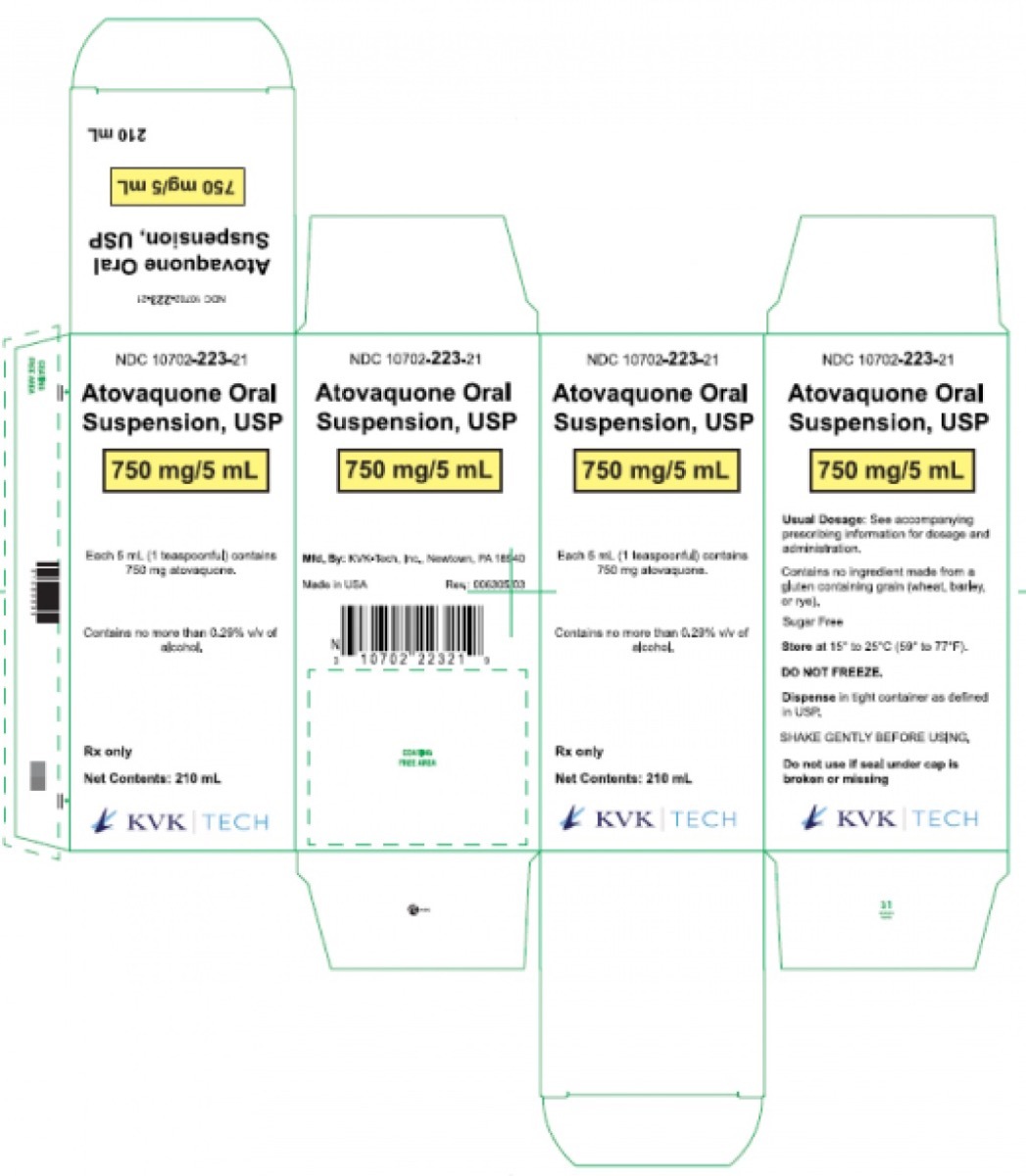
On August 6, the FDA announced that KVK Tech, Inc. had voluntarily recalledTwo a lot of atovaquone oral suspension, USP 750 mg / 5ml.
The affected medicine is reached from 8 oz. bottles with caps resistant to the child. The batch of the affected lots are packed in cartons printed with NDC # 10702-223-21, batch numbers 16653A or 16654A and expiry dates of December 2022.
For the latest health news delivered in your inbox,Sign up for our daily newsletter.
The drug was recalled after the complaints of "unusual gravity".

Atovaquone oral suspension, which is generally used to treat pneumocystist pneumonia Jiroveci - a form of pneumonia that often affects people with HIV and is used in preventing pneumocystis jiroveci pneumonia among immunocompromised persons, is supposed to be protected against Extremely low temperatures.
However, the product has been recalled after several users complained of their exceptionally grantile orders, which could have been attributable to exposure to low temperatures, depending on the recall.
The drug may have been made less effective.

This is not the texture of the medication alone that could have been compromised by its exposure to temperature fluctuations. According to the FDA, while exposure to extremely cold temperatures can affect the taste, texture and appearance of the product, especially, extreme cold can also affect its effectiveness.
While the KVK Tech manufacturer had not received any reports of problems related to the use of the drug recalled at the time the reminder was announced, the recall notice recognizes that "severely immunodepromised patients receiving an oral suspension of Atovaquone less effective can undergo inadequate treatment and infections threatening life. "
If you have the drug recalled at home, do not use it.

If you have medicines from the terrains recalled in your possession, the FDA advice recommends that you stop using it now. The product must be returned to the KVK Tech manufacturer to 110 Terry Drive, NEWTOWN, PA 18940. Customers will be refunded for the cost of their medicines.
If you have reminder questions, contact KVK Tech at 215-579-1842 Ext: 6002 week of the week from 8h to 16h30. Is or at [email protected]. If you have taken the medicine concerned and believe that you have experienced adverse effects, contact a health professional.
RELATED:If you use this medicine, talk to your doctor immediately, "said FDA.

Horror of your wardrobe, or antitrindrends this winter

