The FDA is about to make this announcement of major vaccines, sources say
The change comes after weeks of speculation on the recall shots.
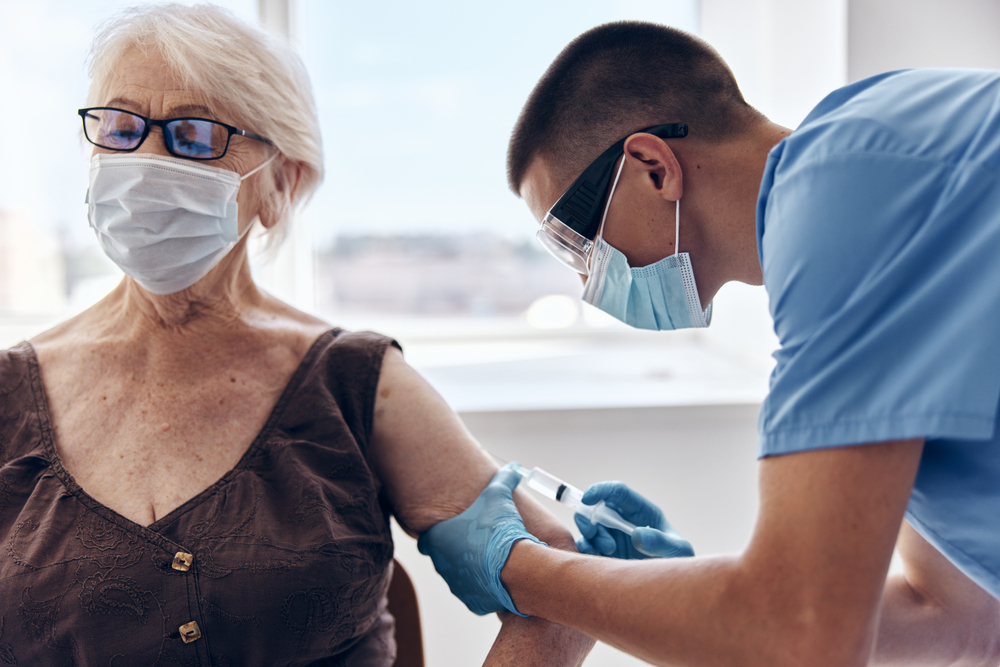
Study after study found that currently available vaccines offer a lot ofProtection against Covid-19. Even in the case of the Delta variant, the shots were considered very effective in preventing serious results or death in the vast majority of cases. But for months, scientists and health experts were interviewed when public servants approve the use of recall shots for those who may need it. Now, sources say that the Food & Drug Administration (FDA) is defined to make a major ad that will allow some immunodezing people to getA third dose of vaccine, NBC News announced first.
RELATED:If you have received this vaccine, you may never need a reminder, a new study indicates.
The change in policy, which should be announced in the month of August, comes after weeks of debate that many weakened immune systems would require a recall to protection against protection against Covid-19, particularly with the highly transmissible Delta variant. . which is currently supposed to be responsible for93% of all cases US data from disease control and prevention centers (CDC) estimate that 2.7% of the US population - or about 9 million people may beclassified as immunocomized, including patients undergoing cancerous treatment, people with HIV and those who have received an organ transplant. However, it is not yet clear that the exact groups would be affected by the announcement of the FDA.
The expected change comes after other countries have begun to offer third parties to those with weak immune responses, including France, Germany and Hungary. It also uses weeks of speculation of the highest American officials that some people of the population would beSoon offered a boost.
"When you examine immune compromised persons, those who have cancers, those of chemotherapy for a variety of diseases, those who have immune depression of some sort of other, they probably never had a good immune response at the beginning, "Anthony Fauci, MD, head of the White House Covid, said at a recent interview with CNN. "We think they should get this extra boost earlier than later. Very quickly," he added.
RELATED:Pfizer Executive says that it is who will first get Covid booster shots.
The assembly search has shown that those with compromised immune systems may require additional shots to generate an adequate immune response that will keep them protected. A great study published in the medical newspaperCancer cell On June 5 found that, while most of the 200 cancer patients included in the study responded well to Covid-19 vaccines, 30% of theseTake immunosuppressive drugs There was no sign of seroconversion, which is the medical term for the production of antibodies in response to a virus. And another recent randomized and placebo-controlled study of Canada found that Moderna vaccine reminder plansgenerated an improved immune response In those who have been given to,The New York Times reports.
RELATED:For more information up to date, sign up for our daily newsletter.
The change in the FDA authorization also comes at a time when non-immunodepromised people started looking for third parties. But both officials have warned that more data were needed before boosters are recommended for the general and insured population that recent research has discovered that the shots are always very effective, even against new variants. As a result, some speculated that it could be months beforeExtra shots become available To those who are not immunocompared, reports of new CBS.
"I still suspect again in September, we can make a more coherent statement about what the recommendation will be here," "Peter's brands, MD, an official FDA top supervises vaccine approvals, said at a webinar in July.
RELATED:If you have modern, it's when a booster will be "being necessary".

17 photos of magnificent Libraries majestic who will make you feel so brilliant

