The FDA investigating the reports of new side effects of frightening ozempic
The agency assesses involuntary reactions associated with popular weight loss drugs.
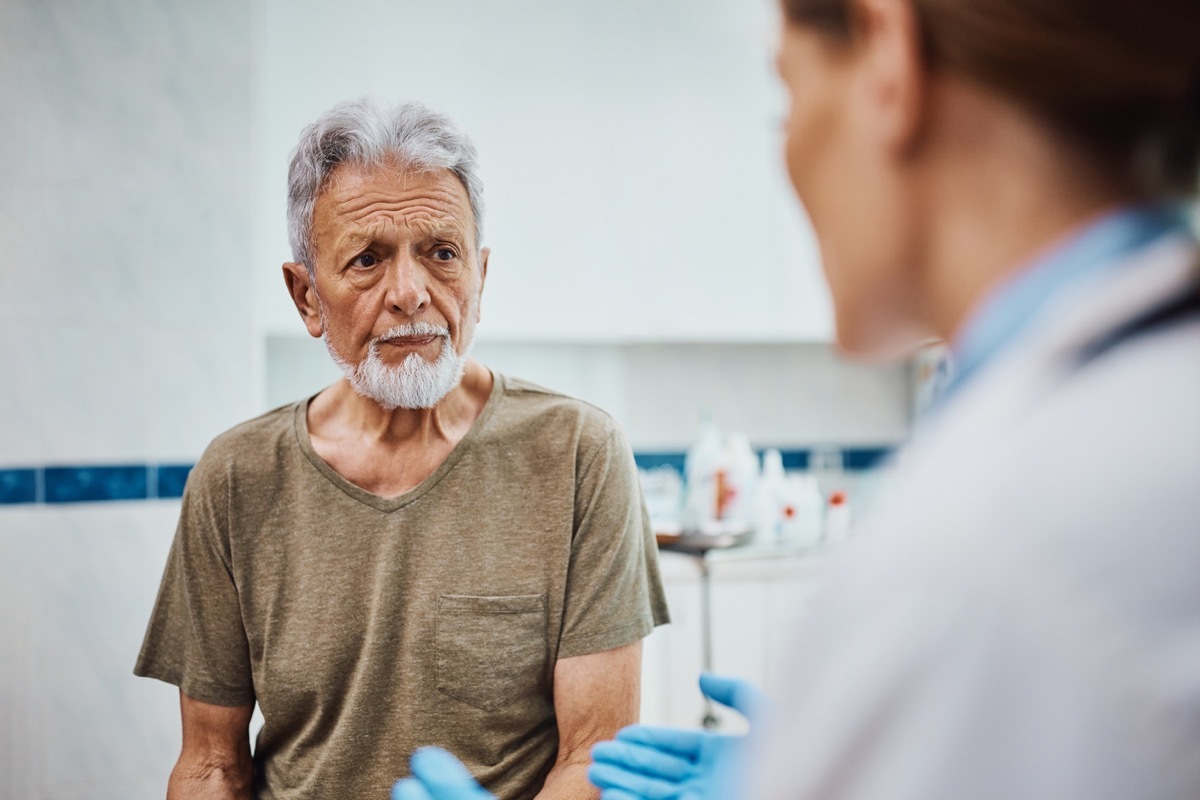
The rise of Ozempic in the past year has been undeniable, but it was not without controversy. While some celebrities praised the drug against diabetes to have helped them drop books, others like Sharon Osbourne And Jillian Michaels warned the hidden dangers to use it. Many people taking ozempic drugs and other similar weight loss products also talked about the unpleasant Side effects They have lived. The most alarming statements have caused a new US Food and Drug Administration (FDA) survey. Now the agency examines some scary effects in particular, which have all been linked to popular weight loss drugs.
In relation: The ozempic patient reveals a new "repulsive" side effect .
In a quarterly report Released on January 2, the FDA revealed that it was examining health problems that could be linked to a group of drugs called GLP-1 receptors. The agency said that it was investigating several semaglutide and shooting drugs on the market - including Ozempic, Wegovy, Mounjaro and Zepbounde - to "potential signals of serious risk [and] new security information identified by the System of reports on adverse events of the FDA (Faers (Faers (Faer). " AE0FCC31AE342FD3A1346EBB1F342FCB
Between July and September 2023, some patients using these weight loss and diabetes drugs sent reports in Faers concerning their experience with three specific side effects: alopecia, aspiration and suicidal ideas. Consequently, the FDA said that it "now assesses the need for regulatory action" with regard to GLP-1 receptors and these reported reactions.
"The appearance of a drug on this list does not mean that the FDA concluded that the drug had the risk listed", FAERS website states . "This means that the FDA has identified a potential security problem, but it does not mean that the FDA has identified a causal relationship between the drug and the risk listed."
In relation: What really happens if you stop taking Ozempic, say the doctors .
As a spokesperson for the FDA Chanapa tantibanchai explained to CBS News , this system helps the agency to monitor "drug safety throughout its life cycle, including after approval", as well as "identify and assess adverse events that did not appear during the process of medication".
It is not immediately clear if these reactions were caused by the drug, underlines the FDA.
"While consumers and health professionals are encouraged to point out adverse events, the reaction may have been linked to the trafficking in the underlying disease or caused by another drug taken simultaneously or has occurred for other reasons, "said the agency.
When these reactions are determined as being caused by the drug, they can lead the FDA to update the drug label or to call for more research on reactions.
"If newly identified security signals are identified, the FDA will determine which actions, if applicable, are appropriate after an in -depth examination of available data," Tantibanchai told CBS News.
In September 2023, for example, the FDA identified several reports of a side effect different from people using semaglutide drugs: intestinal obstruction. Consequently, the agency has published an update Ozempic drug label In September 2023, this mentioned the reports of undesirable effects of Ileus, which is the medical term for intestinal obstruction.
In relation: Ozempic patients say that "stops working" for weight loss - how to prevent this .
Novo Nordisk, which manufactures Ozempic and Wegovy, and Eli Lilly, who produces Zepbound and Mounjaro, recognized the FDA survey on alopecia, aspiration and suicidal ideas in people who used these drugs.
"We are aware that, in the context of surveillance efforts, the FDA assesses several potential signals related to GLP-1 RA drugs and has published information on current assessments on its website," said a spokesperson for Novo Nordisk at CBS News.
The company said that it "works in close collaboration" with the agency to monitor the safety of their medicines.
"Novo Nordisk is held behind the safety and efficiency of all our GLP-1 RA drugs when used as indicated and when taken under the care of an approved health professional," said the door -Sholes.
Meanwhile, an Eli Lilly spokesperson told the media that these concerns are involved after a "rigorous study for many years in clinical trials and a robust approval process" of their drugs.
"Currently, the FDA is examining data on certain potential risks for GLP-1's agonist drugs. Patient safety is our priority, and we collaborate with the FDA on these potential signals," they said.
In relation: For more information, register for our daily newsletter .
Best Life offers the most up -to -date information for high -level experts, new research and health agencies, but our content is not supposed to replace professional advice. Regarding the medication you take or any other health issue you have, always consult your health care provider directly.

12 transformations of celebrities who have disconcerted millions of fans

The woman takes a ultimate revenge on her husband after paralysis
