The new drug has people who lose 19% of body weight, according to research - and it is not Ozempic
Patients who take innovations have lost nearly 40 pounds over 48 weeks.

Although weight loss has always been widely discussed, it has become even more a burning subject in recent months, thanks to the use of the surveys of Medicines like Ozempic . Indicates for the treatment of type 2 diabetes, the Ozempic is often prescribed out of AMM for weight loss, while other similar drugs - such as Wegovy - are actually approved for obesity. Ozempic and Wegovy are manufactured by Novo Nordisk, but it is not the only company to develop treatments that give significant results. Read the rest to find out more about Innovative, a new medication from Eli Lilly & Co. who has patients losing almost 19% of their body weight.
In relation: The new drug has people who lose 60 pounds on average, shows research - and it's not ozempic .
Innovative can have significant weight loss results.

According to a press release on October 29, a result of a New clinical study Show that the innovative (Mazdutide) can have drastic effects on the weight of patients. Innovative - which is not yet approved By the U.S. Food and Drug Administration (FDA) - Targets two receptors, GLP -1R and GCGR, to stimulate weight loss, according to the press release. This is different from drugs like Wegovy and Ozempic, which target only one hormone (GLP-1).
The study included 80 Chinese patients with obesity, those involved in the trial taking nine milligrams of innovation or a placebo. After 24 weeks, patients taking innovatives lost 15% of their body weight, an average of 32.4 pounds (14.7 kilograms), according to the results announced in May.
In total, 59 subjects in the treatment groups and placebo agreed to continue the trial for an additional 24 weeks. After 48 weeks, patients who took innovatives had lost an average of 18.6% of their body weight, or 39.2 pounds (17.8 kilograms) of their weight at the start of the study.
In relation: Countries place new prohibitions on Ozempic-Can the United States follow?
There were additional advantages, the researchers said.
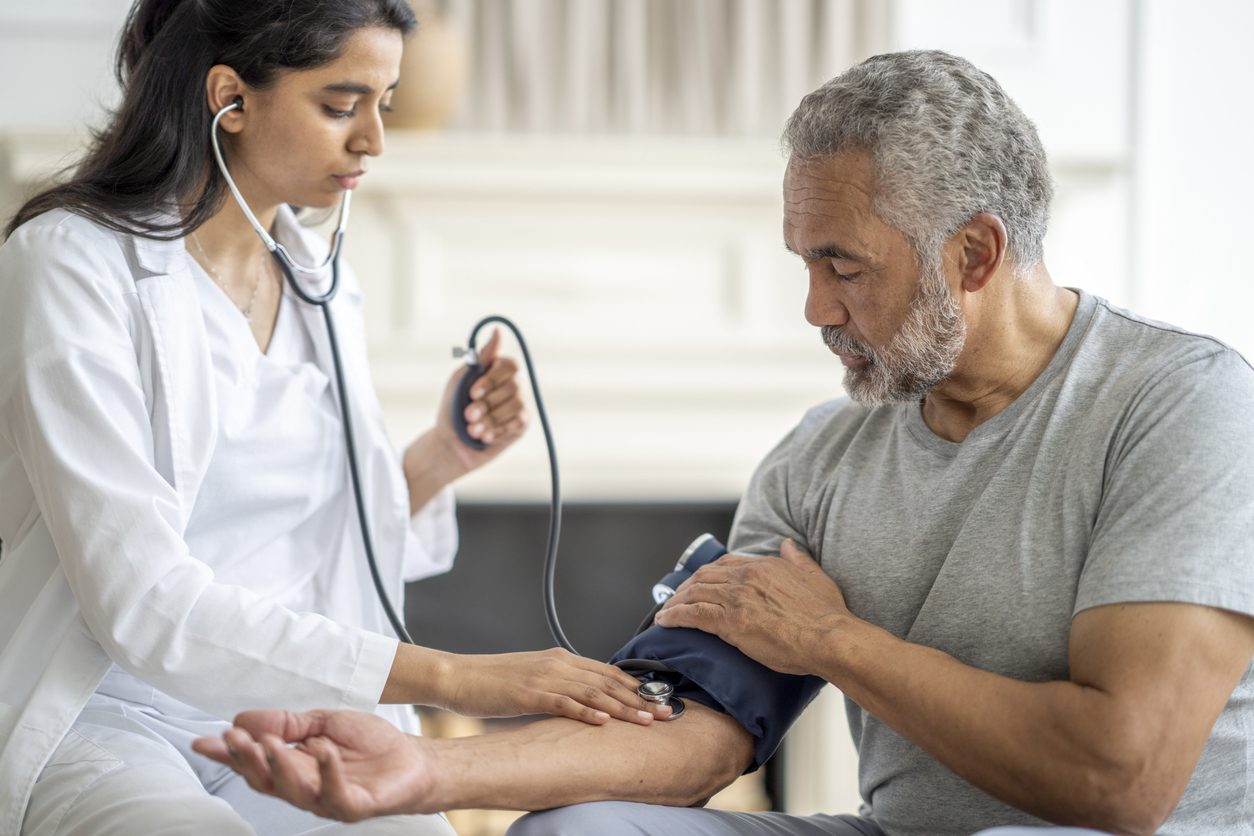
In addition to weight loss, the researchers found that the medium -sized size and blood pressure of patients innovate decreased. Those of drugs have also seen a decrease in liver fat content and cholesterol, according to the press release, indicating wider advantages for people with obesity. AE0FCC31AE342FD3A1346EBB1F342FCB
"As a chronic disease with complex causes, obesity is a significant risk factor for metabolic diseases, cardiovascular and cerebrovascular diseases and tumors", principal researcher of study Linong Ji , MD, director of endocrinology and metabolism at the Popular Hospital of the University of Peking, said in the press release.
Ji continued, "obesity requires long -term treatment and management as well as the attention of the whole company. The 48 -week results of the 9 mg Mazdutide in Chinese subjects suffering from obesity have revealed robust efficiency weight loss of GLP-1R and GCGR double agonists, which is at the forefront of the efficiency of the weight loss of GLP-1 drugs. "
Researchers also noted that no one in the innovative group has stopped treatment due to unwanted events in 48 weeks, and no serious adverse event or increased cardiovascular risk was observed throughout the essay. Regarding side effects, gastrointestinal reactions were the most common, namely nausea, vomiting and diarrhea, which went from light to moderate.
In relation: The ozempic patient reveals "a new" atrocious "side effect .
The results of weight loss are similar to those observed with Mounjaro.
As Jefferies analysts said it to Bloomberg, the amount of weight lost in this test is in accordance with Lost weight on Mounjaro (Tirzepatide), one of Eli Lilly's drugs for type 2 diabetes. Mounjaro should be approved for the treatment of obesity by the end of this year, reported the point of sale.
Earlier this month, Eli Lilly published the results of the clinical trial of phase 3 surmount-3, which assessed Mounjaro's efficiency for weight loss. By October 15 Press release , after a period of diet and exercise of 12 weeks, the patients taking Mounjaro lost more than 60 pounds, or 26.6% of their body weight, more than 84 weeks.
The drug is one of the current studies.

In addition to this study - which is a phase 2 test - there are several other tests in progress to study the effectiveness of lower doses of innovative doses, including four and six milligrams.
The last study phase for the dose of nine milligrams should start at the end of this year. Once the innovative pre-commercialized clinical trials are subjected to the FDA approval.
In relation: For more information, register for our daily newsletter .
Best Life offers the most up -to -date information for high -level experts, new research and health agencies, but our content is not supposed to replace professional advice. Regarding the medication you take or any other health issue you have, always consult your health care provider directly.

Maximum size dresses More: How to choose and how to wear

