The drops for the eyes sold at Walmart and Amazon remembered after 55 reports of infection, the FDA warns
The FDA and the CDC conduct an underway investigation.

The eye drops are A Pharmacy Cabinet Need For many of us - and as spring approach, they are a must if you suffer from hay fever. You could also count on them in winter when you The eyes are irritated or dry Due to the cold.
However, you will want to reveal your supply, because the Food and Drug Administration of the United States (FDA) has just made a reminder for two brands of eye drops. The declines in question, which are sold on Amazon and Walmart, led to 55 reports of infection, loss of vision and even death.
Read the rest to find out which brands are affected and do if you have them at home.
Read this then: If you notice it with your eyes, do your thyroid, doctors say .
Two brands of eye drops are part of the recall.
The FDA and the Centers for Disease Control and Prevention (CDC) study one " largely resistant to drugs "Bacteria strain Pseudomonas aeruginosa , according to a health notice from the Health Alert Network on February 1 of the CDC.
As of January 31, tension was detected in 55 patients from 12 American states: California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah, Washington and Wisconsin. According to a CDC epidemic and patient notification, "common exposure" seems to be artificial tears eye drops - patients reported using 10 different brands , the most common being the artificial tears Ezricare.
In light of the investigation, Global Pharma Healthcare expressed a voluntary recall of its artificial tears Ezricare, LLC and Delsam Pharma, the FDA announced on February 2. Global Pharma is " fully cooperative "With investigators, the company told CBS News." Until now, we have not determined whether our manufacturing plant is at the origin of contamination. However, by abundance of caution, we recall the products in question, "said the company.
Serious infections, vision loss and death have been reported.

After using the eye drops, patients reported inflammation of the eyes, respiratory infections, urinary tract infections and sepsis, said the CDC. In some cases, these conditions have led to more serious results, including permanent vision loss, hospitalization and death linked to blood infection.
The specific tension of Pseudomonas aeruginosa The bacteria are called metallo-β-lactamas mediated by Verona Integron (VIM) and spectrum-β-lactamase extended in Guiane (GE), which is shortened to Vim-Ges-CRPA. The strain has never been reported in the United States so far, according to the CDC.
Until now, bacterial contamination has only been detected in open bottles of Ezricare of two states. The CDC said that contamination could have occurred while the drops were used or during the manufacturing process. The agency is currently testing unprecedented bottles of artificial tears from Ezricare to confirm if it could have happened during manufacturing.
Also on February 2, the FDA warned customers not to buy Ezricare or Delsam Pharma Artificial Tears, explaining that Global Pharma Healthcare Private Limited, based in India, was in violation of the regulatory agency Good current manufacturing practice (CGMP) due to a "lack of appropriate microbial tests, formulation problems ... and a lack of appropriate controls concerning the stimulation-activator packaging. The company was placed on import alert, according to the FDA, which means that these products cannot enter the United States.
In relation: For more up-to-date information, register for our daily newsletter .
If you have these eye drops, stop using them immediately.

According to the CDC, bacteria Pseudomonas aeruginosa is a germ found in soil and water , and it is generally distributed in health environments. It can infect different parts of your body, including your eyes and lungs. Making things more complicated, these infections become more difficult to treat due to antibiotic resistance, which means that the germs do not respond to drugs trying to kill them. AE0FCC31AE342FD3A1346EBB1F342FCB
The CDC and the FDA say that you should stop using all the drops for the reminded eyes - and that if your health care provider has recommended brands, to contact them for an alternative.
The CDC invites you to be proactive if you display signs of an infection. "Patients who have used Ezricare artificial tears and who have signs or symptoms of an eye infection, such as eye discharge, eye pain or discomfort, redness of the eye or Eye -eyelid, the feeling of something in the eye, increased sensitivity to light, or blurred vision, should ask for a timely medical care, "said health advice.
However, if you have used these products and have no symptoms, the CDC does not currently recommend that you see your health care provider.
Here is how to identify the products recalled.

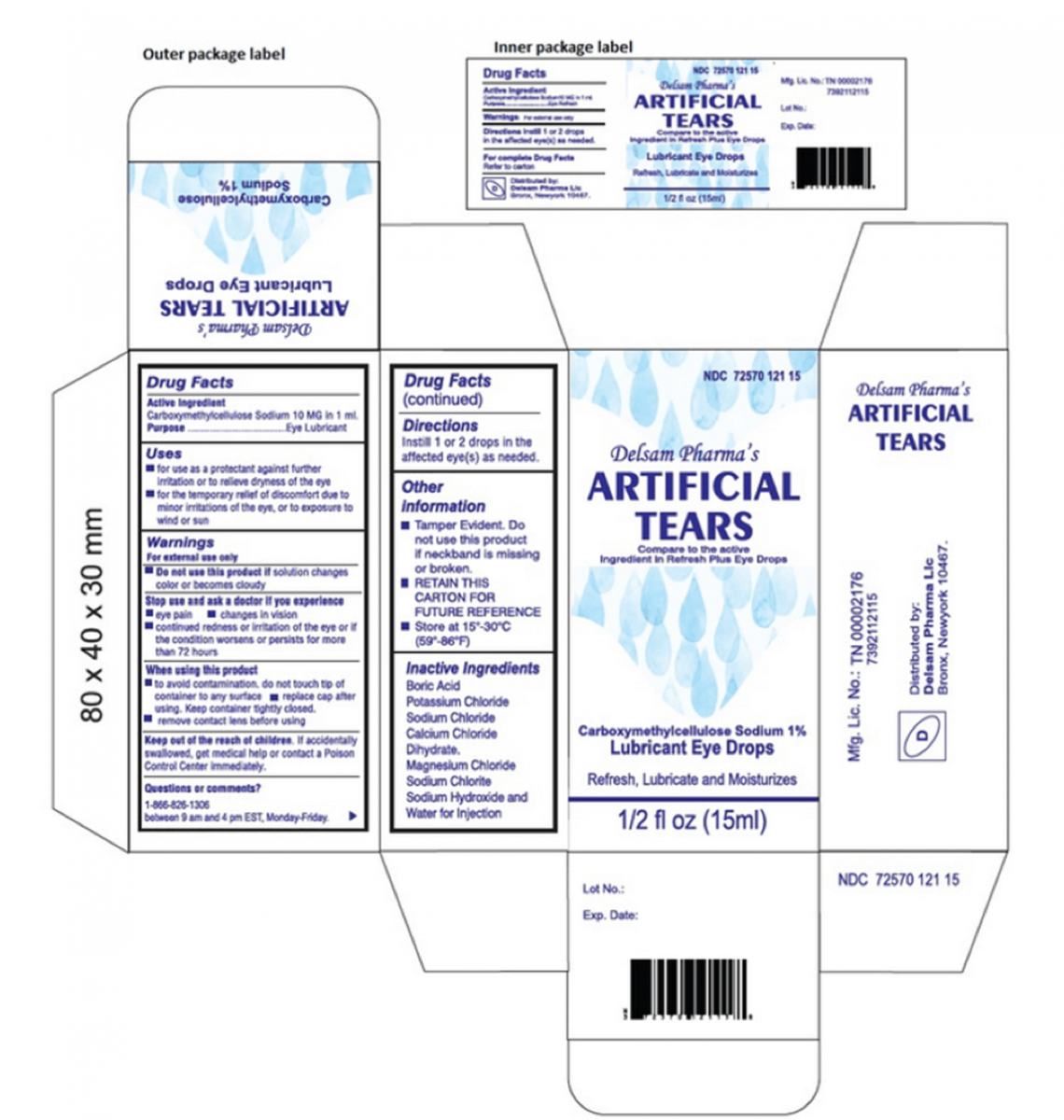
According to the FDA, the recall applies to all the many artificial tears of Ezricare and Delsam Pharma. The artificial tears of the eye drops, which are used to prevent irritation or to relieve drought or discomfort, had a safety seal and were sold in cardboard boxes on the counter.
The artificial tears distributed by Ezricare have a National Code of Medicines (NDC) of 79503-0101-15 and a universal product code (UPC) of 3-79503-10115-7. Those distributed by Delsam Pharma have an NDC of 72570-121-15 and an UPC of 72570-0121-15. The UPCs are printed under the barcode at the bottom of the box, while the NDCs are printed in the upper right corner at the front of the box.
Artificial tears have been sold online in the United States, the reminder announces. They were "obtained via Amazon and Walmart", confirmed a CDC spokesperson at CBS News, as well as during visits to hospitalized and ambulatory patients. The drops of Ezricare were also one of the most sold ocular drought ins on Amazon last year, reported the point of sale.
Global Pharma Healthcare informs the two distributors of the problem, requesting that wholesalers, retailers and customers cease to use the products. You can report unwanted reactions to the FDA Medwatch of the FDA events online program ,, by email , or by fax.

Fauci says that's what is the most "disturbing" on the recent CVIV-19 figures