These skin care products can cause a "permanent injury," said the FDA in a new warning
The agency calls on the counter remedies "potentially dangerous".
For several people,Take care of their skin is essential to their daily routine. And for everything, imperfections compared to water -moving head imperfections, it can take a wide range of products to ensure that all the steps are covered. Finding the right items for your skin type can sometimes take tests and errors, the worst result being generally that it is ineffective or causes slight irritation. But now, the Food & Drug Administration (FDA) warns the public of specific care products that could lead to "permanent injuries". Read the rest to see the elements you need to avoid from now on.
Read this then:Never take this popular OTC medication for more than 2 days, warns the FDA.
The FDA has a specific process to ensure the safety of care products.

Like all the food supplements you see for sale in the United States, skin care products aresubject to a meticulous examination Federal agencies like the FDA to ensure that they are sure to use. However, this also means that the items you see on the shelf are not required to obtain a "prior pre-application" before putting on sale and can only be regulated for violation of a list of rules established by Federal food, drugs and cosmetics Act (FD & C Act).
The products go back to this law when they commit "falsification" - which refers to the use of ingredients or potentially dangerous contaminants - or "bad eternity", referring when a product is "false or deceptive" On its advantages or does not include all the necessary information on its label.
"Companies and individuals who manufacture or market cosmetics have legal responsibility to ensure the safety of their products," writes the FDA on its website. "Neither the law nor the regulation of the FDA requires specific tests to demonstrate the safety of individual products or ingredients."
Now, some products have been criticized by the agency and could face legal action.
The FDA warns against certain care products on the market.

On August 10, the FDA issued an alert to the public that he hadSent warning letters At Amazon.com, Ariella Naturals and Laboratories justified for the introduction and sale of Tags and Skin Tags moves which have not been assessed by the FDA for safety, efficiency or quality, and require the Approval of the FDA ". In addition, the agency claims that the products also violated the FD & C law when companies presented them for interstate tradewithout obtaining appropriate approval.AE0FCC31AE342FD3A1346EBB1F342FCB
"It is the FDA's obligation to protect public health against harmful products not approved for the American market".Donald D. Ashley, JD, director of the compliance office at the FDA medication assessment and research center, said in a press release. "The agency's rigorous surveillance strives to identify threats to public health and prevent these products from reaching our communities. Do not sell products that violate federal law. ""
The agency claims that companies will now have 15 days to respond with the measures they have taken to deal with violations, declaring that "warning letters are not supposed to be a final solution from the agency".
RELATED:For more up-to-date information, register for our daily newsletter.
The agency warns that items could cause "permanent damage" if used.
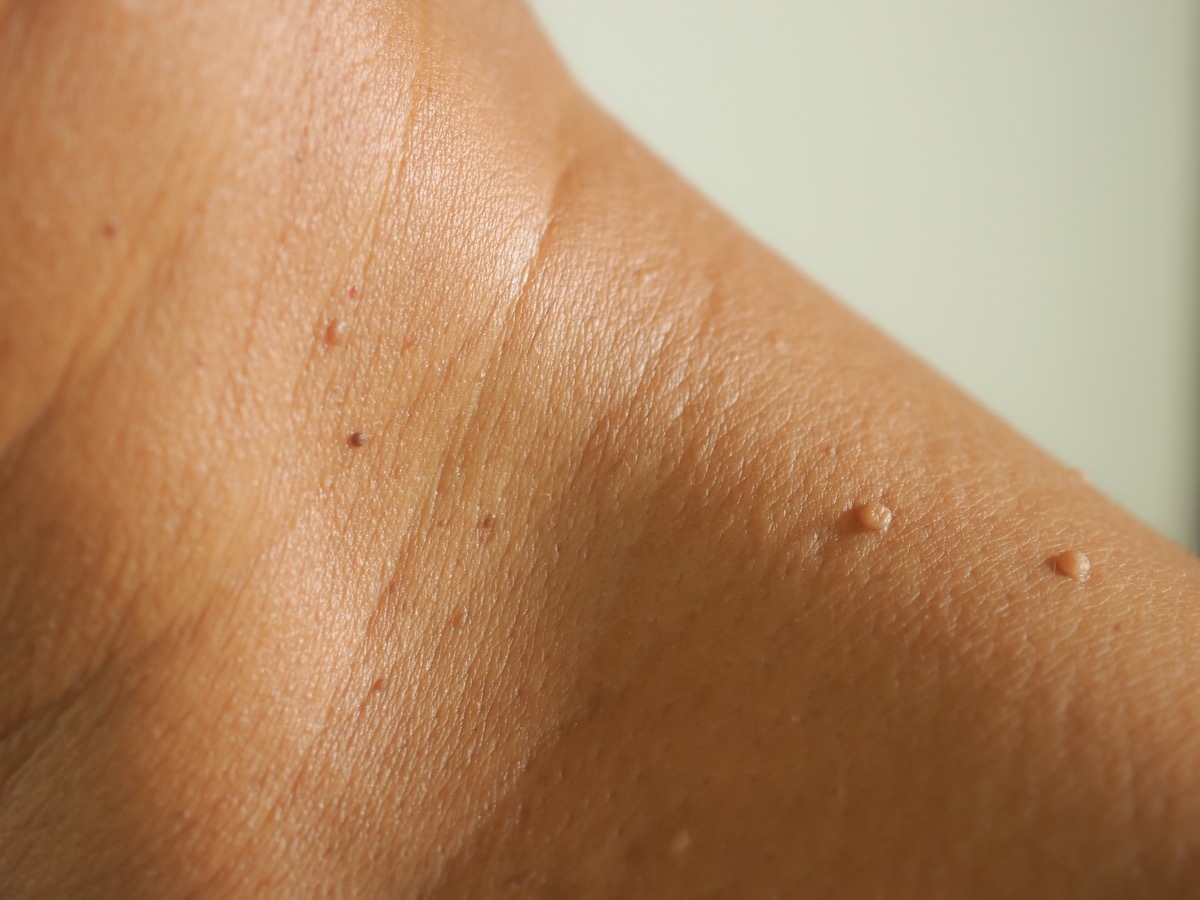
The agency says that it advises to use moving tags of taupe and skin due to "potentially harmful side effects and serious risks" which they pose, in particular "skin injury, an infection requiring antibiotics, Scars and diagnosis and treatment of delayed skin cancer ". The FDA says it has also received consumer reports which "have developed permanent skin injury and infections" due to the use of over -the -counter remedies (OTC).
"The products marketed for the aesthetic elimination of moles of moles, skin labels or other skin lesions generally contain high concentrations of salicylic acid or other potentially dangerous substances," writes the agency in its opinion. "These products often do not remove the lesion or do not withdraw all this. Worse, even if the lesion falls, the product can cause permanent wounds to the surrounding skin, such as scars or discoloration. In some cases, the Final result can be more painful and notable than the original lesion, especially if you apply the product to your face. "
The FDA advises to see your doctor to check any taupe or suspicious skin label and says that customers must "avoid" over -the -counter moves.

The FDA advises the public "to avoid these products" because of the potential danger they represent. Instead, the agency says that a doctor must always check the tapes or skin labels to ensure that stains are not cancerous and that any potentially necessary treatment is not delayed. Although most tapes and skin growths are not worrying, the FDA warns that there are some signs that they could be more serious.
"If a taupe or a skin label develops, changes, bleeding or is painful, you should consult a doctor," warns the agency. "Do not treat the skin problem yourself. If you remove it or change your appearance, health care providers may have trouble determining if it is skin cancer and Propose an effective treatment plan. "
Anyone who has undergone an undesirable event following the use of moving tags or skin tagscan also report it to The FDA security and adverse event information program by filling out the online form. You can also call the 1-800-332-1088 for the form to be sent to you or download it before completing it and sending it to the address indicated or fax it to 1-800-FDA-0178.

17 magical foods that relieve the symptoms of the cold

