We are also near a vaccine, according to the doctor who runs research
Now that large-scale clinical trials have started, here's the Covid vaccines here.

With coronavirus, still continuing to rump in many parts of the United States, thepressure for a vaccine that protects against COVID-19 Built, driving scientists to work tirelessly in a race against the clock. And according toThe New York Times, researchers can beA step closer to have a vaccine readybefore the end of the year, which would be an unprecedented execution time.
"Have a safe and efficientVaccine distributed at the end of 2020 is a prolonged goal, but it's the good goal for the American people "Francis Collins, MD, Director of National Institutes of Health (NIH), said in a statement.
On July 27, one of the first large-scale studies to test the safety and effectiveness of aCOVID vaccine officially started. The specific vaccine was developed by NIH, in collaboration with Biotech Company Moderna Inc. and involves 30,000 volunteer participants,The New York Times reports.
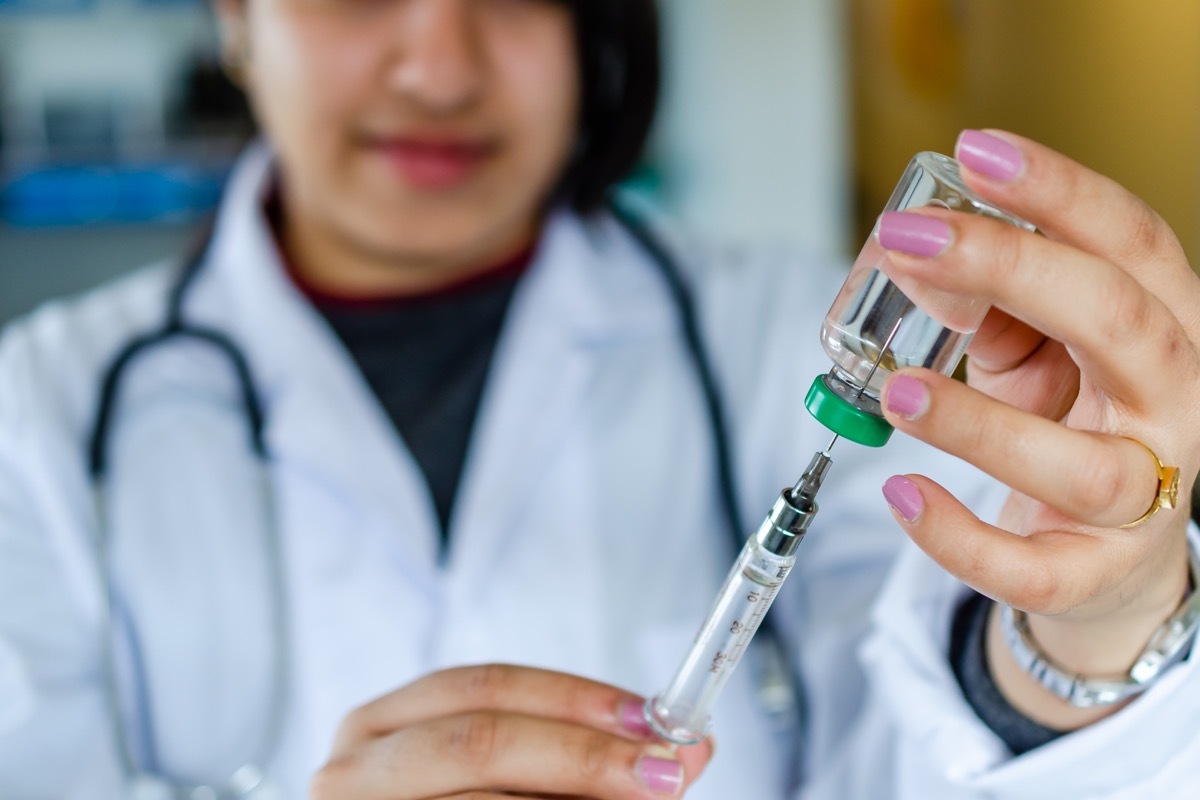
Half of the participants (15,000)will receive two doses of the vaccine-With 28 days between each shot and half will receive a placebo of salt water solution. Neither the volunteers nor health professionals who do not manage injections know only patients get the real vaccine and that they receive placebo, according to the associated press (AP). Following the injections, the scientists of the study will closely follow each group of participants to see what other infections when it comes to their daily lives - it is particularly important for those ofareas where the virus is still spreading significantly, ap.
RELATED:For more information up to date, sign up for our daily newsletter.
In their observations, scientists will also retain narrow tabs on all subjects of side effects. And while the main purpose of the tests is to find proof of whether the vaccine may or may not prevent COVID-19,The New York Times reports that the study will also try to discover "if this can prevent serious CIVID-19 and death; if this can completely prevent infection, based on laboratory tests; and ifjust a shot can prevent the disease. "
Modern, for one, feels confident with what they brought to the table. "We are optimistic, carefully optimistic,"Stephen Hoge, MD, President of Massachusetts Moderna, told a commission of the White House last week, the AP reported. And when asked when his company will be able to prove the effectiveness of the vaccine, Hoge said: "Towards the end of the year." And for more interesting vaccination news, checkDo this only thing could drop the risk of your Alzheimer by 30%.

Fly these 16 secrets of mental health of famous geniuses

