If you use this rinse-mouth, the FDA says to stop immediately
The FDA has just warned an oral rinse reminder that could affect your morning routine.

Before joining your medicine cabinet in the morning, you need to take care of your oral hygiene, you must give an extra look to your mouth. Americans are advised to check their oral rinse after aThe major reminder has been published on a mouthwash commonly prescribed toPatients with gingivity. According to a recall notice posted by the Food and Drug Administration (FDA), the product in question, gum paroex, can be contaminated by bacteria. Read it to find out if your morning routine is affected and for another reminder to be aware, checkIf you have this spice in your pantry, the FDA says check it immediately.
Read the original article onBetter life.
It is a popular prescription mouth bath that has been recalled.
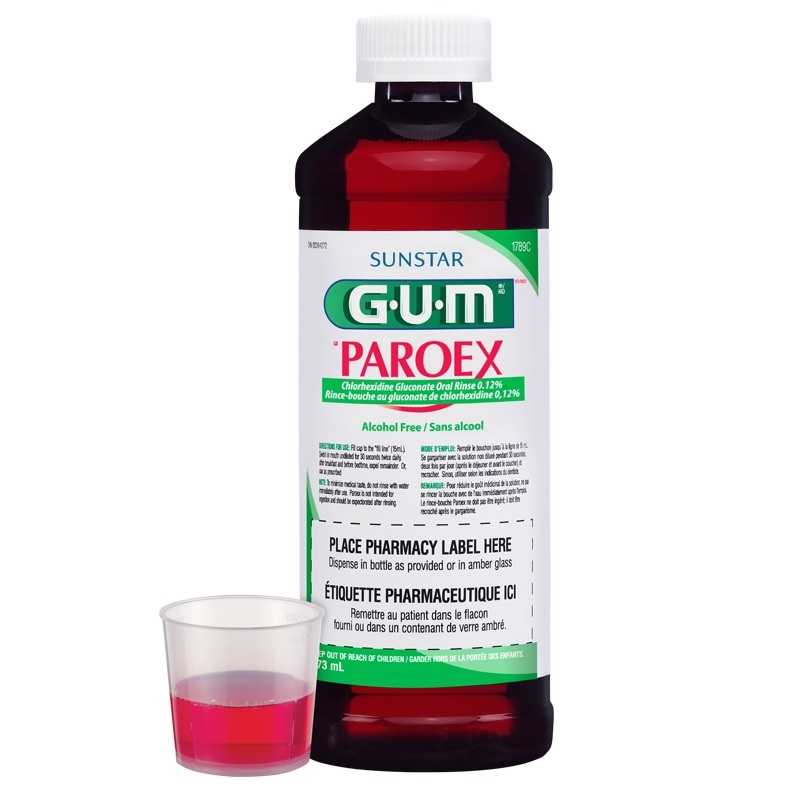
The rinse-mouth in question is the Gum Paroex® Chlorhexidine Gluconate Oral Rinse USP, 0.12%, manufactured by Sunstar Americas, Inc. (SAI) of Schaumburg, Illinois. Gum Paroex is distributed nationally through dental offices, pharmacies and pharmaceutical wholesalers to prescribe to patients.
The potentially contaminated products are in a cylinder of amber and marked 1789p (in a bottle of 473 ml) and 1788p (118.25 ml bottle). The expiration dates on the mouthwash in question went from December 31, 2020 to September 30, 2022.
It's aexpansion of the reminder of the mouthwash Sai had published at the end of October. For another medicinal reminder, you need to know, checkIf you have this guest medicine, you have to check your bottle immediately.
The mouth bath is particularly harmful for those who are immunocompared.

The concern is that the paroex gumThe mouth bath can be contaminated with the bacteriaBurkholderia Lata,Which, if consumed by a person who is already immunocompared, can lead to oral infections requiring antibacterial therapy. Among the particularly vulnerable patients, there is an additional risk of potentially fatal infections such as pneumonia and bacteremia. According to the FDA report, 29 adverse mouthpiece reactions have been reported to date. And for another type of danger that could be hidden in your home, checkIf you have this fan at home, stop using it immediately.
And it is particularly dangerous for COVID-19 patients.

"The use of contaminated paroex on patients with pre-existing respiratory conditions, including those infected with COVID-19, is particularly dangerous," said Sai in its recall.
And for more regular recall news,Sign up for our daily newsletter.
The FDA says to stop using the mouth bath immediately.

According to the FDA, "patients, pharmacies and health facilities in possession of these products should stop using and dispense immediately." Patients must also contact their doctor if they feel bad effects.
Sai is currently notifying its customers to organize returns of recalled products. And for other dangerous products that could be at home, checkIf you have these meats in your refrigerator, eliminate them immediately.

Kirsty Alley said that his relationship with Patrick Swayze was "worse" that a case

