The only thing people who get blood clots after the vaccine have in common
Here's what you need to know about declared blood coagulation cases.
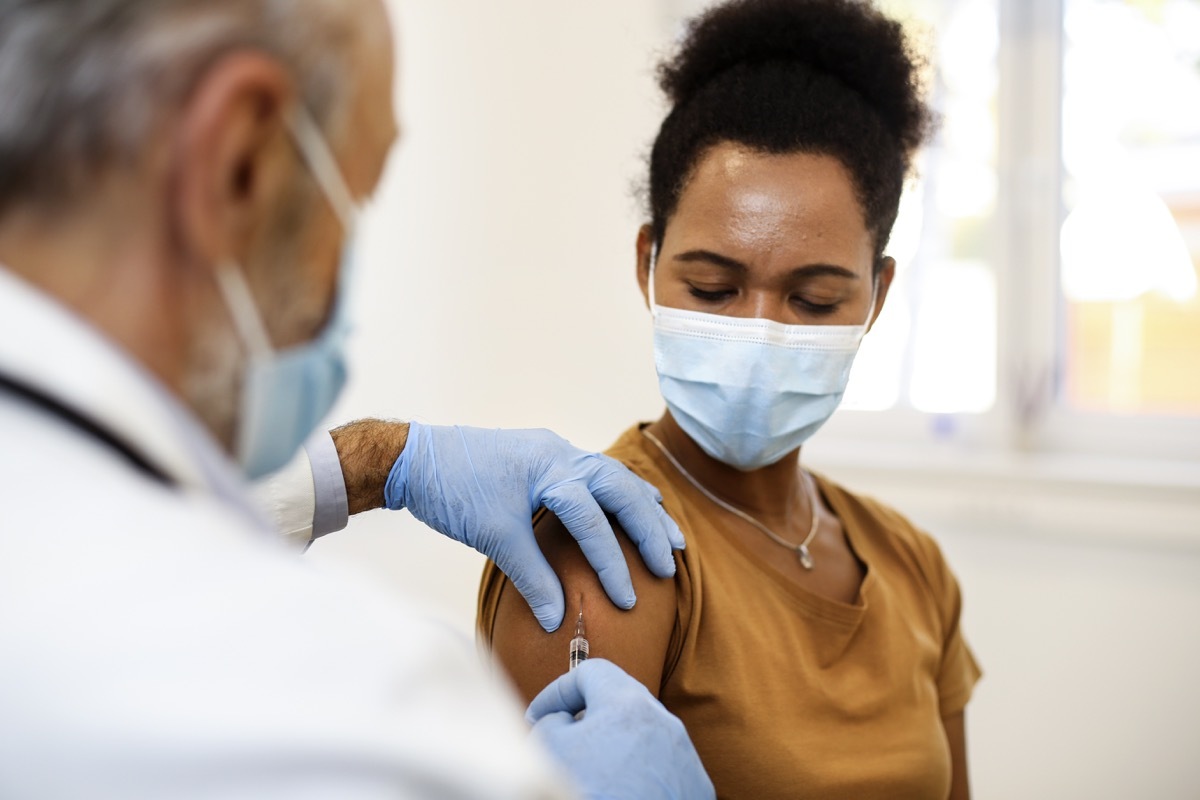
Reactions must be expectedAfter a Covid vaccineAs indicated by disease control and prevention centers (CDC), since your body works strongly to strengthen immunity. However, the increase in relationships of certain very rare but dangerous reactions, such as blood clotting, have made people more and more worried in recent weeks. The agencies began to examine the blood coagulation reports along two different Covid vaccines: Johnson & Johnson and Astrazeneca. When you look at the data, there is a clear common point between the small number of people who have blood clots after these vaccines. Read on to find out what these beneficiaries have in common and for more side effects of vaccine,Moderna provoked this reaction in 82% of people, the new study says.
The cases of the most reported blood clots have been in women under the age of 60 within three weeks of vaccination.
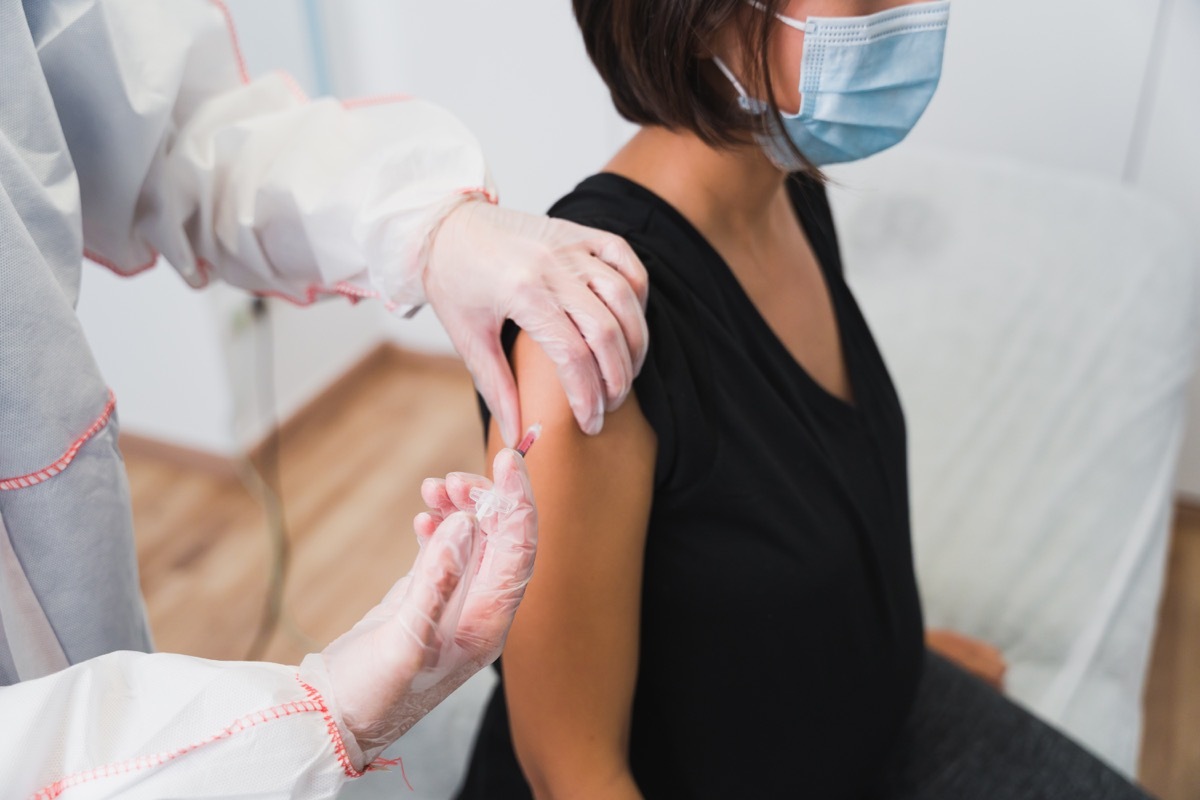
Six people haveExperienced blood cabot After receiving the Johnson & Johnson dose vaccine at the US Johnson & Johnson,The New York Times reported. The six were women aged 18 to 48, who developed blood coagulation in one to three weeks after vaccination. Blood coagulation has also been reported as a rare reaction as a result of the AstaZeneca Covid vaccine, which is not yet available in the United States according to the European Medicines Agency (EMA), most of theseCases took place in women Less than 60 years in the two weeks after vaccination.Christian Bogdan, member of the German vaccine committee, said Reuters that theRisk of blood coagulation The highest in women aged 20 to 59, observed between four and 16 days after receiving the Aztrazeneca vaccine. And for more reactions,If 1 of these 3 body parts begin to swell after your vaccine, call a doctor.
Blood coagulation is always a very rare reaction as a result of the vaccine.
It is important to note that these are extremely rare reactions. As Reuters highlights, the risk of women who earn oral contraception is four out of 10,000 - a much higher number than seen as a result of these COVID vaccines. According to the CDC, almostSeven million people received Johnson & Johnson vaccine at the USS. And only six people would have been victims of blood clotting. In a statement of April 13, Johnson & Johnson said they are aware of an "extremely rare disorder involving people with blood clots in association with low platelets in a small number of individuals" who received their Covid vaccine.
On the same rating, only 169 cases of blood clotting according to the AstaZeneca vaccine have been reported to the EMA on 34 million doses administered. "The combination of blood clots and low blood platelets is very rare and the overall benefits of the vaccine in CVIV-19 prevention outweigh the risks of side effects," said EMA in its report. And for more information up to date,Sign up for our daily newsletter.
The CDC and the FDA recommended a break in the use of Johnson & Johnson.
CDC and Food and Drug Administration (FDA) are met to publish a statement recommending aImmediate pause on Johnson & Johnson vaccine according to these blood coagulation ratios. "We recommend a break in the use of this vaccine on abundance of caution. This is important, in part, to ensure that the community of health care providers is aware of the potential of these adverse events and planning a Appropriate recognition and management due to single treatmentRequired with this type of blood clot"," said the agencies in a declaration released on April 13.
The CDC said that this will meet on April 14 to further examine these cases of blood clotting and "assess their potential importance", while the FDA states that it will examine and also examine these cases. Agencies recommend HALL HALLT providers using this vaccine until the end of the survey process. However, they also note in their statement that "right now, these adverse events seem extremely rare." And for more CDC advice,The CDC says not to take this after your vaccine without OK from your doctor.
If you notice specific symptoms after vaccination, you should request medical care.

States can always choose to give Johnson & Johnson vaccine, although some have already announced that they will comply with the break. If you receive or received this vaccine, you must know some symptoms that may indicate that you know the rare coagulation reaction. According to the CDC and the FDA, if you received Johnson & Johnson and "develop serious headaches, abdominal pain, leg pain or shortness of breath in the three weeks after vaccination", you must contact your supplier immediately health care. And for more information on vaccine reactions,Do this after your vaccine can make side effects worse, doctors say.
CDC and FDA has just made this shocking vaccination recommendation

