If you take this medicine, American officials have a new warning for you
If you have this medicine at home, it's time to take preventive measures for your loved ones to be safe.

You probably do your best forKeep your family members safe at homeIf you keep secure sharp knives or make sure your cleaning products are safe for use around the family animal. However, even if you are judicious of the obvious risks around your home, you may miss - and it's right in your medicine cabinet. Read it to find out if a medicine you are taking could put your health in danger. And for more security risks are hiding for sight,This popular device could put your family at risk, finds studies.
On 18 March, the Consumer Products Safety Board (CPAC) announced that the manufacturerGenentech had recalled about 14,000 prescription drug units evrysdi. The drug used to treat muscle atrophy of the spine (ADM) in children and adults, was packed so that bottles can flee and lead to inadvertent exposure to prescription drug in contact with Skin or eyes.
The reminder medicinal product is delivered in 100 ml. Bottles and "Evrysdi (Rispiplam) for the oral solution" and "NDC 20242-175-07" printed on the label. Although no injuries related to the product has been reported at the time of recall, there were 26 rebuilt ratios fleeing. If you have the medicine concerned at home, the CPSC recommends "Immediately [Store] Evrysdi in a safe place and the view of the children", and if you notice that your bottle is leaking,Contact Genentech for a replacement. If the bottle of your possession flees and comes into contact with your skin, the CPSC recommends washing with soap and water; If this comes into contact with your eyes, use water to rinse them.
Genentech is not the only company that had to draw recently its market products. Read it to find out which other prescriptions and supplements you should be depart from your medicine cabinet now. And if you want to protect your family from the fur family,If your pet wears this pass, they could be in danger.
1 Spironolactone tablets

On March 9, the Food and Drug Administration (FDA) announced thatBryant Ranch Prefack Spironolactone tablets have been recalled due to a potential packaging error. According to the notice of reminder, the 25 mg of the brand. and 50 mg. Tablets may have beenput in the wrong packing, possibly leading to some people getting half of the necessary dose and some get their regular dosage twice. Those who get doubled from their dose could experience potentially deadly potassium surge, while those who become half "can undergo an elevation of blood pressure or increased swelling caused by excess liquid. , reports the FDA. If you have the medicine assigned to the house, you can contact Bryant Ranch preparative at 877-885-0882 from Monday to Friday between 6:30 and 6 pm.; The company can also contact you. And if you want to keep safe,If you have this soup in your refrigerator, write it, says USDA.
2 Dr. Reddy Drugs
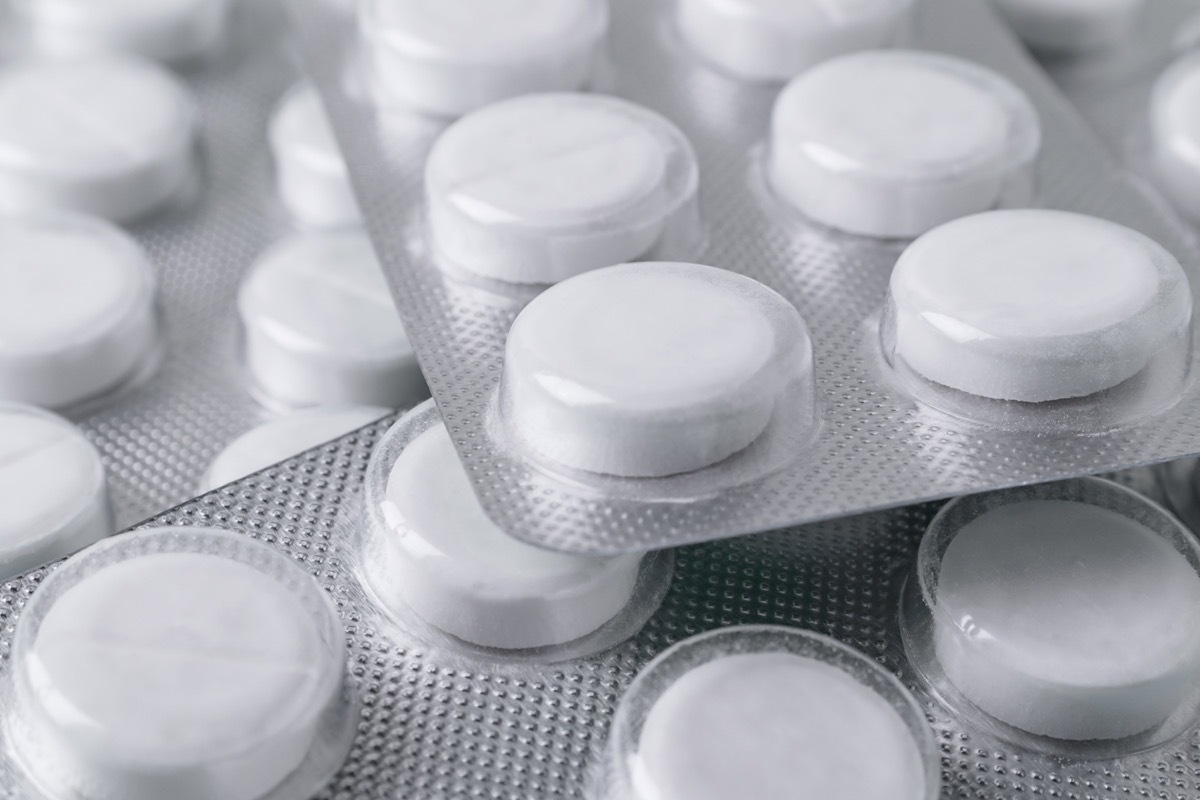
On February 21, CPSP announced that about 21,400 packages of packed medications inDr. Reddy Blister Packs had been recalled Due to concerns, the packaging was not sufficiently sure of the child. TheAssigned drugs Include 100 mg mesylate tablets of the mark, 400 mg mesylate tablets of imatinib, 50 mg of pregabaline capsules, 75 mg of pregabaline capsules, 100 mg of pregabaline capsules, 150 mg of pregabaline capsules, 800 mg of sevelamer carbonate tablets, 5 mg of Tadalafil tablets and 20 mg tadalafil tablets and "can pose a risk of intoxication if the content is swallowed by young people children ", according to the notice of reminder. If you have any of the drugs concerned in your possession, the CPSC recommends storing them in a place where they are not accessible by children and contact Dr. Reddy's at 888-375-3784 orin line for a refund. And for the latest reminder news delivered directly to your inbox,Sign up for our daily newsletter.
3 Secret Supplements of Adam

On February 16, theFDA announced voluntary recallthe additional force of Adam 500 tablets and the additional force of Adam 3000 tablets, two supplements of masculine improvement, because of concerns they could beContaminated with Sildenafil and / or Tadalafil, active ingredients in erectile dysfunction drugs on prescription Viagra and Cialis, respectively. The added ingredients could critically in a critical blood pressure in nitrate drugs or could cause health complications ranging from stomach pain to sudden death in otherwise healthy people. If you have the supplements at home, the FDA recommends that you stop using them and contact [email protected] to organize their return; The company also contacts customers.
4 Numb cream
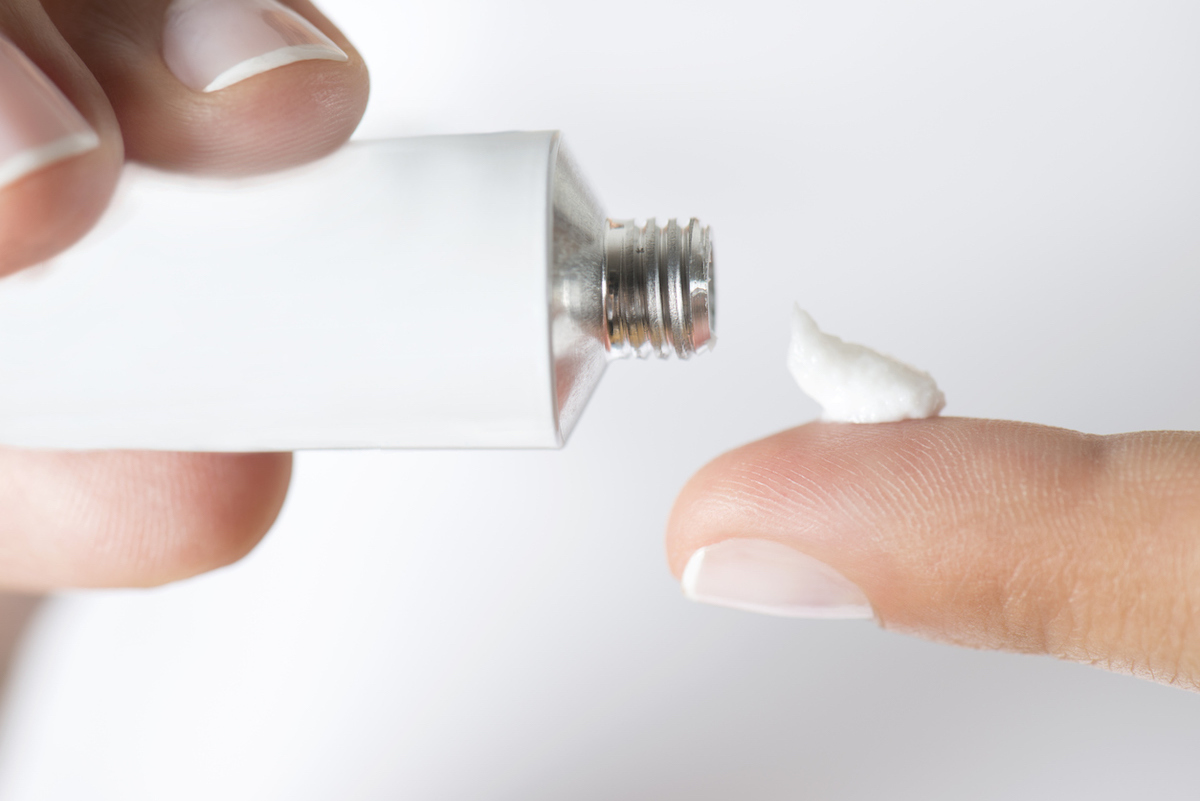
Think about the application of numb creamBefore your vaccination? You may want to check to ensure theSomeone you use has not been recalled first. On January 14, CPSP announced that about 10,000 tubes ofScalpa's maximum resistance topical anesthetic cream had been recalled On unprofessional packaging to children, which could present a risk of child poisoning. Anyone with the recalled cream at home canContact Scalpa; The brand also tends to customers regarding returns. And if you want to protect yourself and your loved ones,If you have one of these 2 popular cars, do not park in the garage.


