The CDC has just changed this important Covid vaccine guidance guide
The recent update provides more advice for people with certain pre-existing conditions.
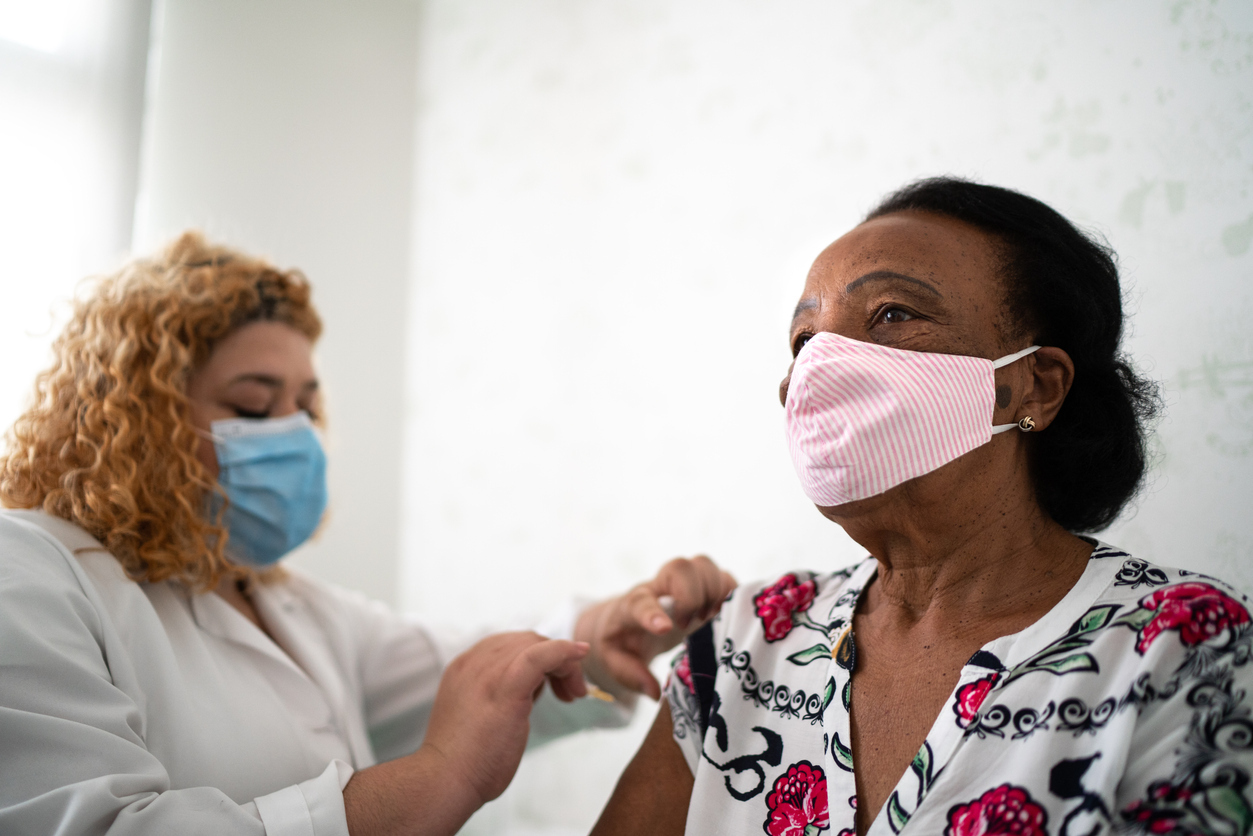
The release ofEffective coronavirus vaccines in mid-December represented a major step in the pandemic that seized the pandemic that seized the world for the best part of 2020. And while health agencies and medical experts were controlled for the safety of the public vaccine, its deployment Initial saw a handful of isolated incidents that led doctors to "monitor" those with certain pre-existing conditions. But a new update of US disease and prevention control centers (CDC) hasmodified a guideline for people With these conditions, saying that it was safe for them to receive the Covid vaccine as long as they "did not have a serious allergic reaction to any of the ingredients" used in doses.
Newly-developed COVID vaccines use mRNA molecules instead of a weakened or inactivated virus, allowing the body to produce the antibodies necessary to combat disease safely from becoming potentially infected, such as with traditional inoculations.
Now, the updated CDC guidelines published on December 26, specify that ARMA's Arna Covid-19 vaccines developed by Pfizer and Moderna canto be managed safely to people which suffer from specific diseases, conditions or previous health problems. Read on to see what pre-existing conditions have been highlighted in the update and for moreshould notGet the two doses of the vaccine, checkThese are the only people who should not get 2 doses of the Covid vaccine.
Read the original article onBetter life.
1 People with weakened immune systems
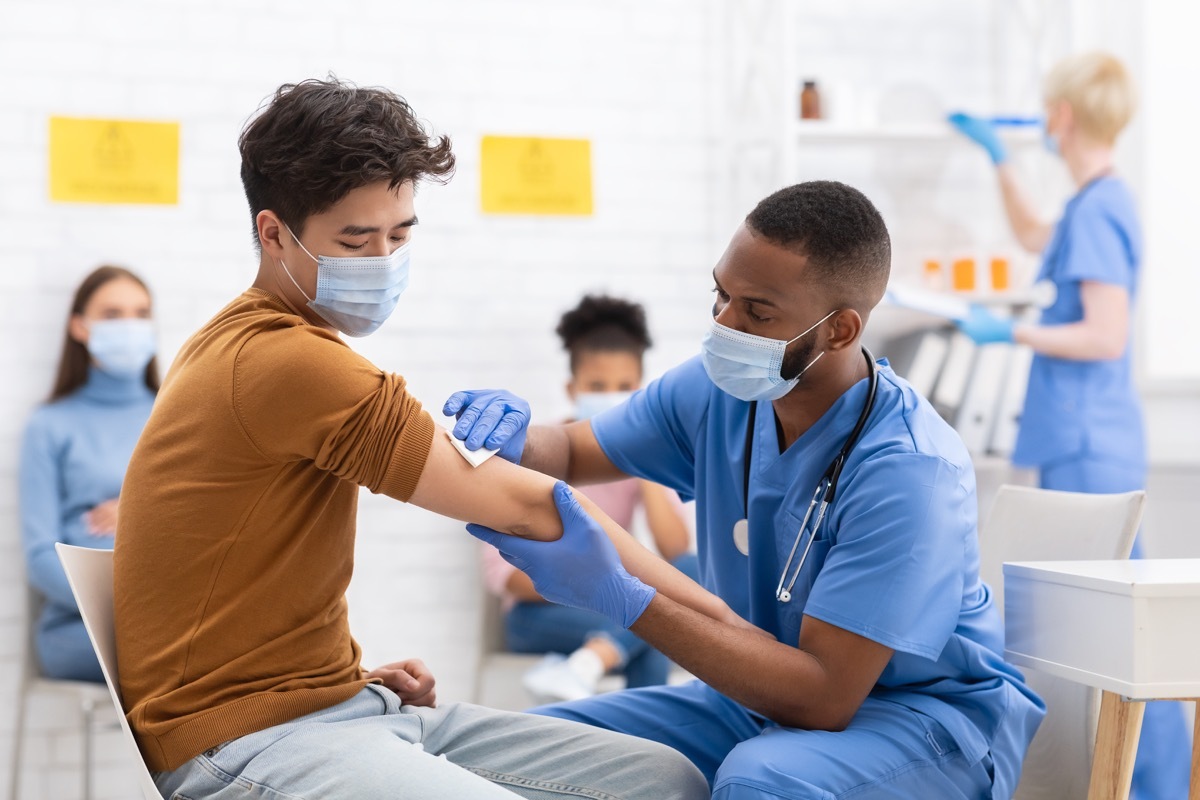
The CDC emphasizes that, as a group, which is statistically at a higher risk of developing serious COVID-19, those that are HIV-positive or have an weakened immune system due to drugs or other diseases may still receive safely A coronavirus vaccine. The Agency points out that people with HIV were included in the original clinical trials for Pfizer and Moderna, but that specific data of the group have not yet been published.
The guidelines warn that "people with weakened immune systems should also be aware of the potential for reducing immune responses to the vaccine", exhorting anyone with this condition to continue to follow the basic health guidelines such as wearing a mask, Social distancing and a diligent hand the laundry. And for more updates on Covid,Sign up for our daily newsletter.
2 People with autoimmune conditions
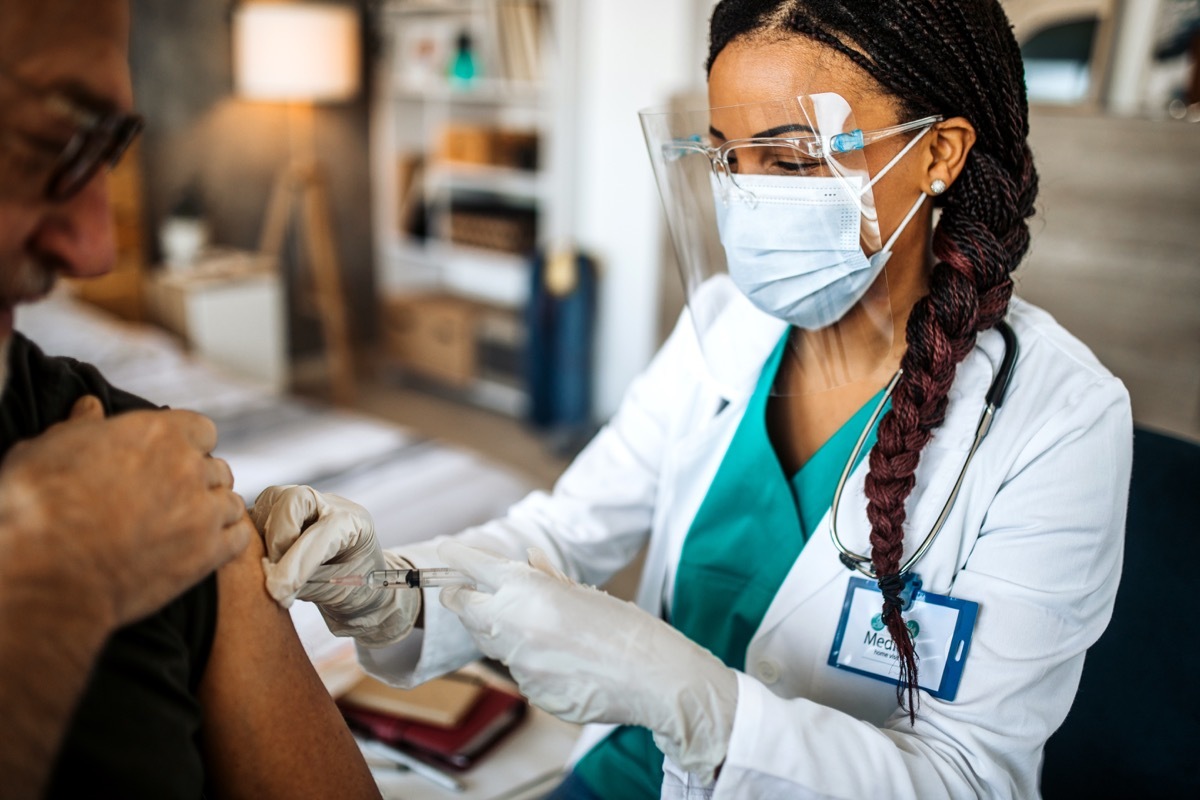
Anyone suffering from a self-immune disturbance has also been cleared to securely receive theVVID-19 vaccine by the CDC. The Agency stresses that participants in these conditions were also eligible to participate in initial clinical trials, but similar to those with HIV or weakened immune systems, isolated data on its effects have not yet been published. And for more information on how to prepare your vaccine, checkThe unique effect of Dr. Fauci is worried with his next shot of Covid.
3 People who had Guillain-Barre Syndrome
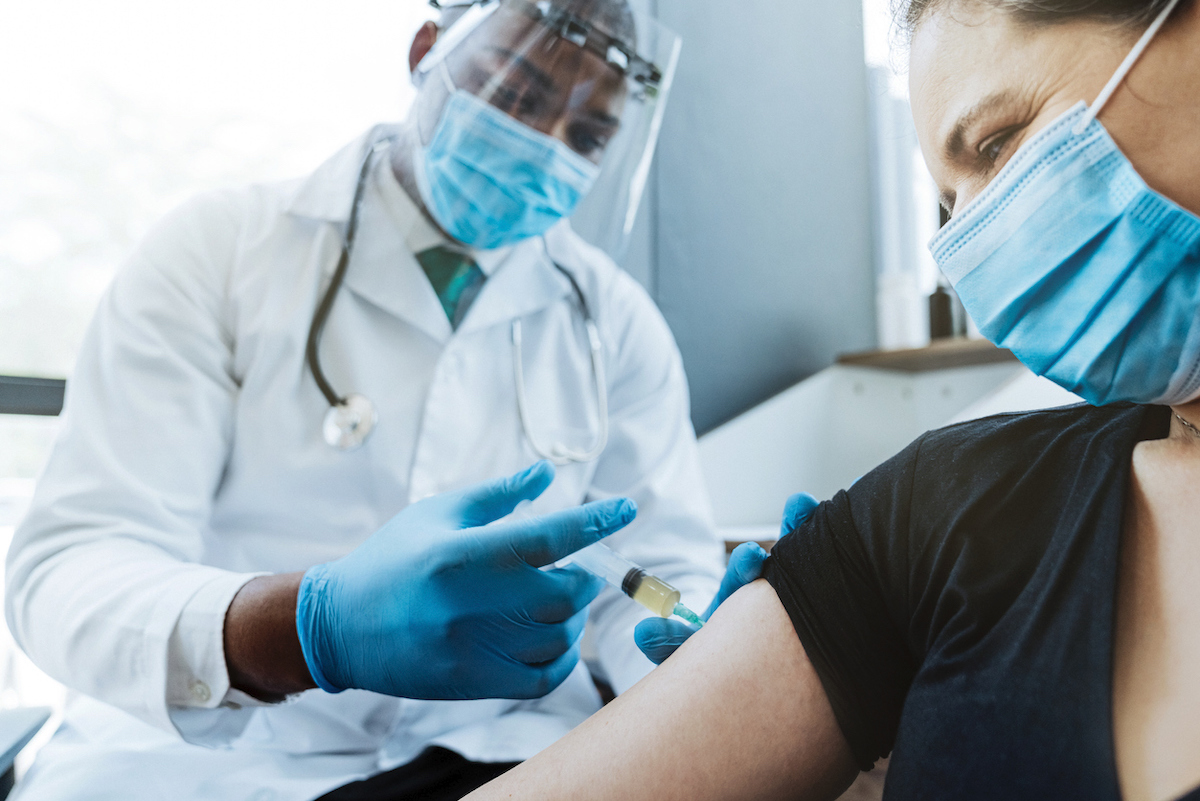
Although its true causes are unknown, Guillain Bar's syndrome is a condition in which theThe immune system attacks nerves throughout the body, create numbness and paralysis in patients. The vast majority of patients recover and their brush previous with the disease does not prevent them from being vaccinated against Covid-19.
"To date, no case of Guillain-Barre Syndrome (GBS) has been reported as a result of the vaccination of participants in clinical vaccine clinical trials mRNA Covid-19", the status of CDC guidelines . "Within a few exceptions, the Independent Advisory Committee on Immunization Practices (ACIP)General Guidelines for Best Practices for Immunization Do not include a history of GBS in order to precaution with vaccination with other vaccines. " And for more information on the vaccination process, checkThese 2 states go against the Vaccine Recommendations of the CDC.
4 People who had Bell's paralysis

In clinical trials,Very isolated incidents of Bell Paralysis-A temporary paralysis of muscles on one side of the face - werereported as a side effect At some participants. However, the guidelines emphasize that "the Food and Drug Administration (FDA) does not consider that these are greater than the expected rate in the general population", adding that "they have not concluded that these cases have been caused by vaccination ". The CDC asserts that it erases anyone who may have already been affected by the disease to obtain the vaccine safely. And for more facts about the vaccinated being, consultDr. Fauci simply demystated the 4 biggest myths on the Covid vaccine.

FIND POLICE ABOUT FOREIGN STUDENT LAVISH LIFESTYLE

