If you use this to sleep, talk to your doctor immediately, say manufacturers
This popular product could put your health in danger.

Whether you are a pill or comfortable configuration under weighted coverage, countless people use a wide variety of prescription and non-prescription products to help them get a healthier and more restful sleep. However, if you use a particular sleep accessory, you can put your health in danger, its manufacturers say. Read it to find out if you should stop using this Nighttime Essential now.
RELATED: If you take this medicine common to sleep, stop now, a new study says.
Philips issued a reminder on specific CPAP and BIPAP devices.
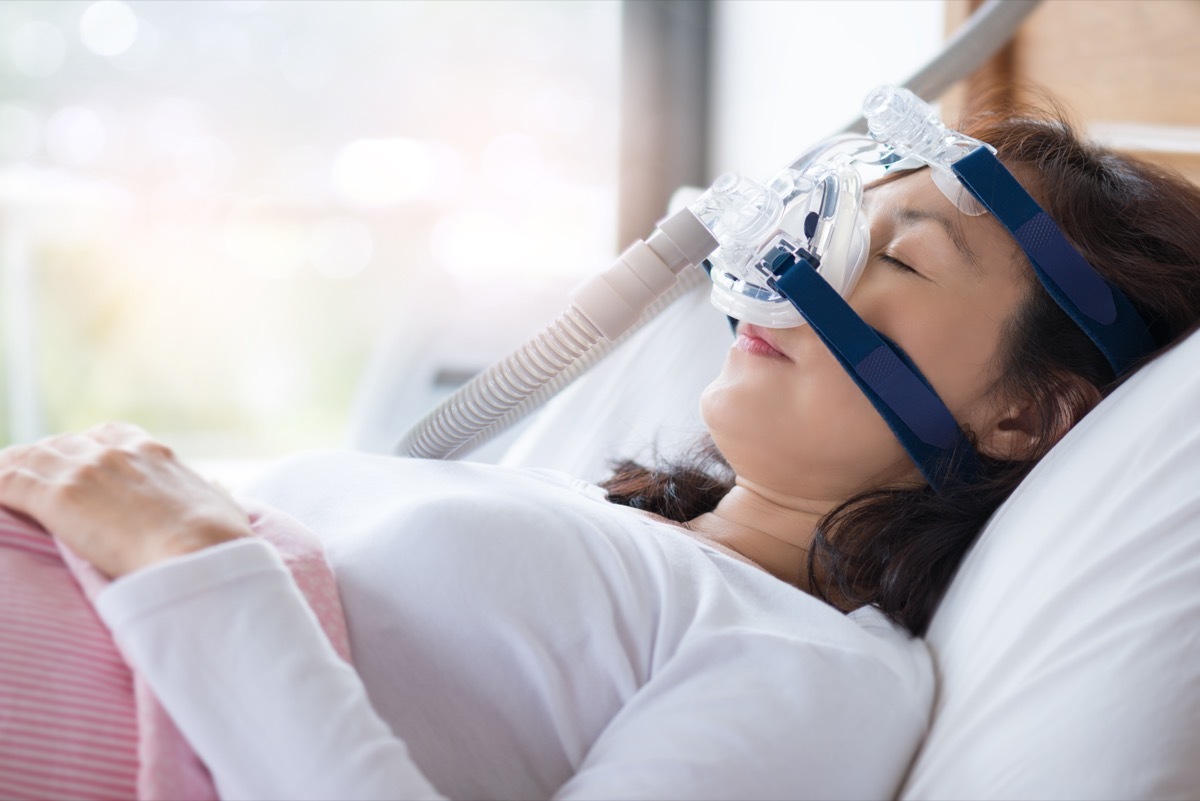
June 14, Philips Royal Philips Electronics Manufacturerissued a voluntary reminder Pressure Pressure Pressure Machines Specific (CPAP) and Bi-Level Pressure (BIPAP), usually used to treat sleep apnea.
The devices concerned include ASV DreamStation; Dreamstation ST and Avaps; Systemone ASV4; C series asv, s / t and avaps; Omnilab Advanced Plus In-Lab Titration Device; System a series Q; Dreamstation CPAP, CPAP Auto and Bipap; Dreamstation GO CPAP and APAP; DORMA 400 and 400 CPAP; and Remstar self-cpap. All recalled CPAP and BIPAP devices were carried out before April 26, 2021 and all series numbers of the aforementioned models are recalled.
For the latest recall news sent directly to your inbox,Sign up for our daily newsletter!
The reminded devices could present a serious risk to health.

According to Philips, the use of recall products could potentially cause damage to your health because of the type of polyester-based polyurethane foam type inside the devices.
"The risks include that the PE PE foam can be degraded into particles that can enter the airway from the device and be ingested or inhaled by the user, and the foam may die in certain chemicals. The degradation of the Foam can be exacerbated by unapproved use. Cleaning methods, such as ozone, "explained the company in a declaration, noting that high humidity and high heat can accelerate the degradation of the foam.
Although there were no deaths related to the use of one of CPAP or BIPAP machines recalled at the time of reminder announcement, Philips indicates that the use of devices can contribute to health problems, Including "headaches, irritation, inflammation, respiratory problems, etc. Possible toxic and carcinogenic effects "due to exposure to foam particles. Foam gassage can contribute to" headache, irritation, hypersensitivity, nausea / vomiting and possible toxic and carcinogenic effects. "
If you use one of the affected machines, talk to your doctor immediately.

If you use one of the CPAP or BIPAP machines recalled, Philips recommends that you "stop using your device and work with your doctor or provider of sustainable medical equipment (DME) to determine the most appropriate options for Continuous treatment ".
However, if you choose to continue using the device, PHILIPS always recommends discussing the issue with a health professional to determine whether its continued use is more beneficial than prejudicial. If you have one of the machines affected at home, Philips works with regulators to replace foam that has been identified as a potential health risk.
Philips also recalls a number of his fans.
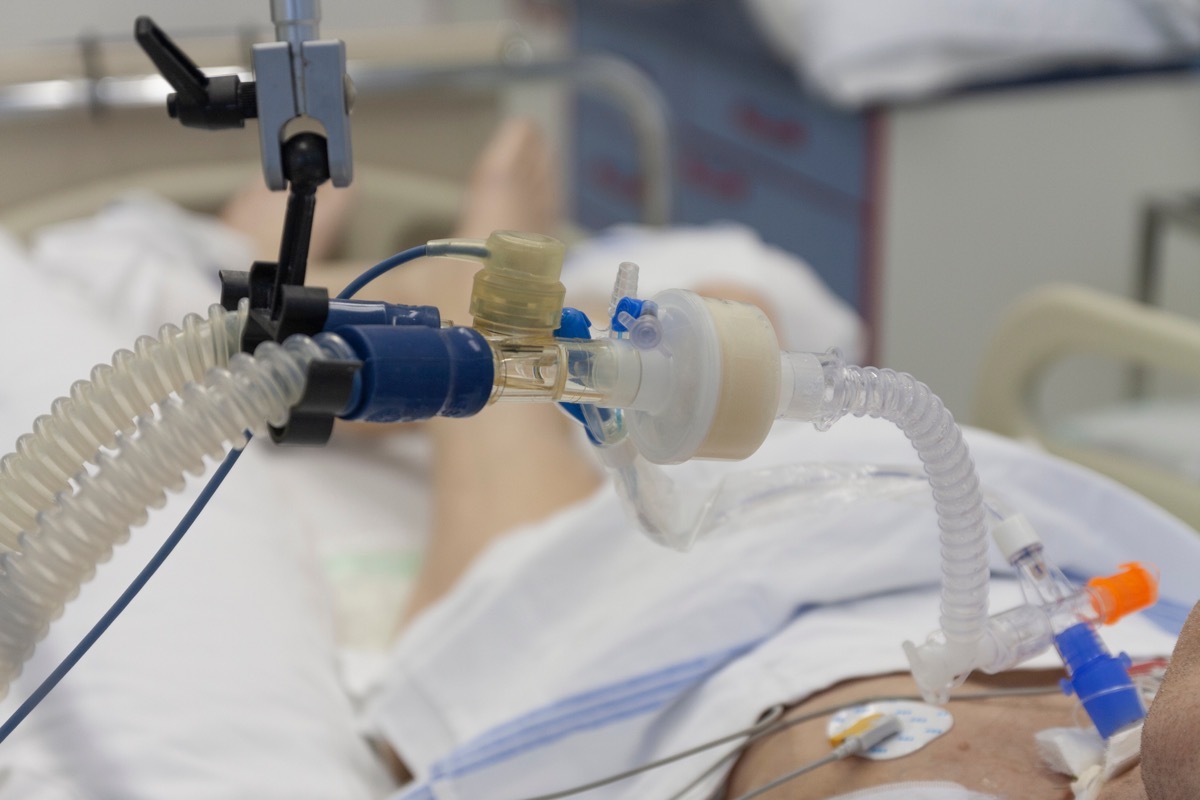
In addition to the assigned CPAP and BIPAP machines, Philips also released a reminder of several fans due to the use of potentially carcinogenic foam in their design.
The affected models include the E30; Trilogy 100; Trilogy 200; Garbin Plus, Aeris and Lifevent models; A-hybrid bipap series A30; A-series BIPAP V30 Auto; A-series BIPAP A40 series; and BIPAP A30 and A series. Medical facilities and suppliers in possession of recalled devices can visit thePhilips reminder site Record the products concerned and receive additional instructions from the manufacturer.
RELATED: Never do this when you shower at night, doctors warn.