If you are prescribed any of these drugs, do not use them, says FDA
Several batch of prescription drugs are drawn from the market on serious safety concerns.

If you try to get quarantine healthier, you are not alone. While for some people, it means hitting the gym once again again or eating a healthier diet, for others, it means compensating for the lost time when it comes to medical appointments, you have Maybe missed because of the rental.
Unfortunately, if you have recently been prescribed two types of specific medicines from your doctor, your health could be in danger. Read it to discover the prescription medications that the US Food & Drug Administration (FDA) indicates that you have to stop using now.
RELATED:If you use this to sleep, talk to your doctor immediately, say manufacturers.
InnovIx Pharmaceuticals, Inc. recalled all sterile compound drugs.

On July 13, the FDA announced that Innovaix Pharmaceuticals, Inc. had recalled all itsSterile composite drugs.
Assigned drugs include selolein / ipamorelin 3mg with batch number SIP210 and expiration date 12/15/2021, Lot number SIP215 and expiry date 01/14/2022 and Lot number SIP220 and expiry date 01/23/2022; and aod-9604 3 mg with lot number AOD205 and expiration date 11/09/2021, Lot number aod210 and expiry date 11/18/2021, lot number AOD 215 and expiry date 12/15 / 2021 and lot number AOD202 and expiration date 11/09/2021. Medications are contained in packed glass bottles in 3 by-3-inch white boxes.
For the latest health and safety news delivered directly to your inbox,Sign up for our daily newsletter!
Medications may have serious health risks.
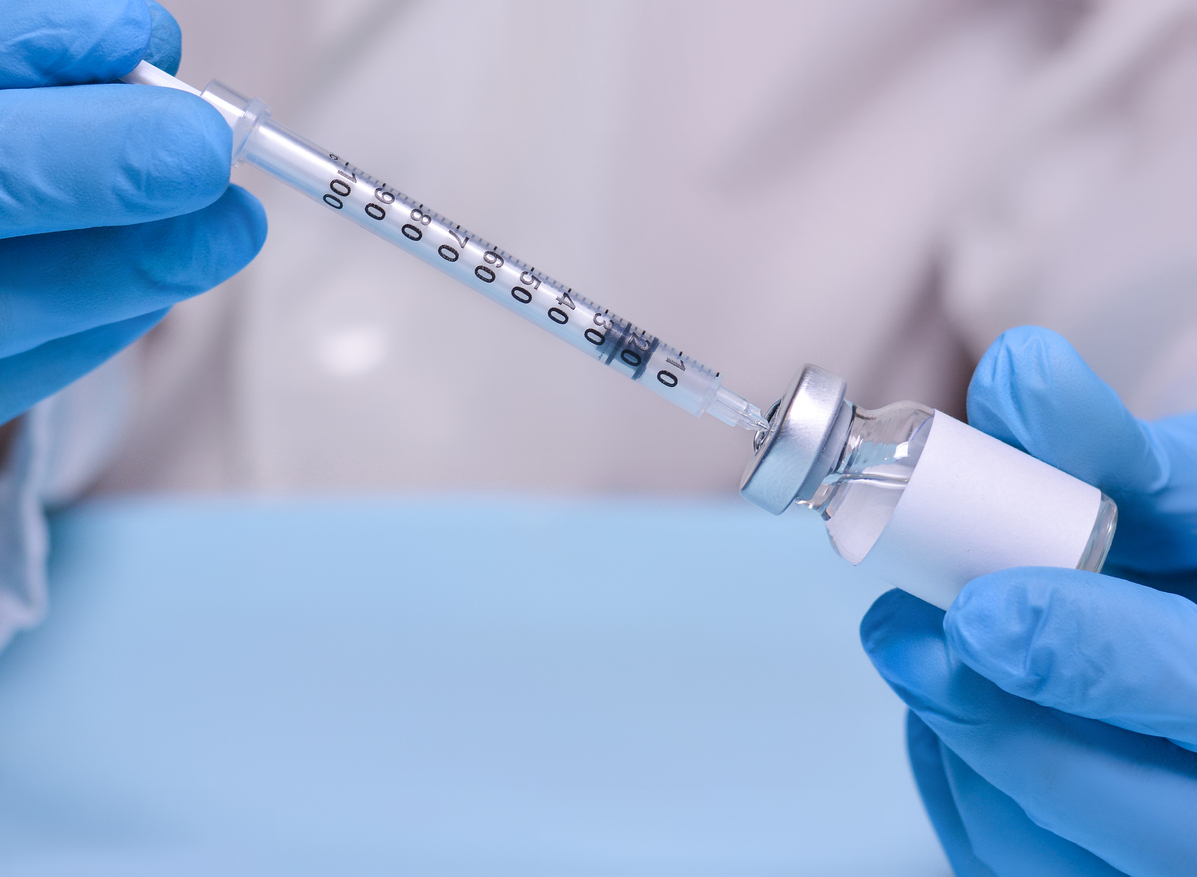
Drugs, which are often used to increase the production of human growth hormones and stimulate metabolism, among other uses, have been drawn from the market because of their concerns about their lack of sterility.
Sterility problems have been discovered during a routine FDA inspection and could put these prescribed risk drugs. "The administration of a drug product intended to be sterile, which is not sterile, could result in serious infections likely to threaten life," explains the FDA.
If you have medicines at home, do not use them.

Although, from the date of recall, no state of adverse effects related to the use of products, the FDA indicates: "Customers who have received sterile compound products submitted to the voluntary reminder should not use them and return the Produced at the pharmacy for a full refund. "
If you have any reminder questions, contact Innovix Pharmaceuticals, Inc. at 800-370-1910 weekdays from 9h to 16h30. CST, or email to the company of [email protected]. If you think you have experienced harmful effects related to the use of these drugs, contact a health professional.
This is not the only reminder of the company solely because of infertility problems.
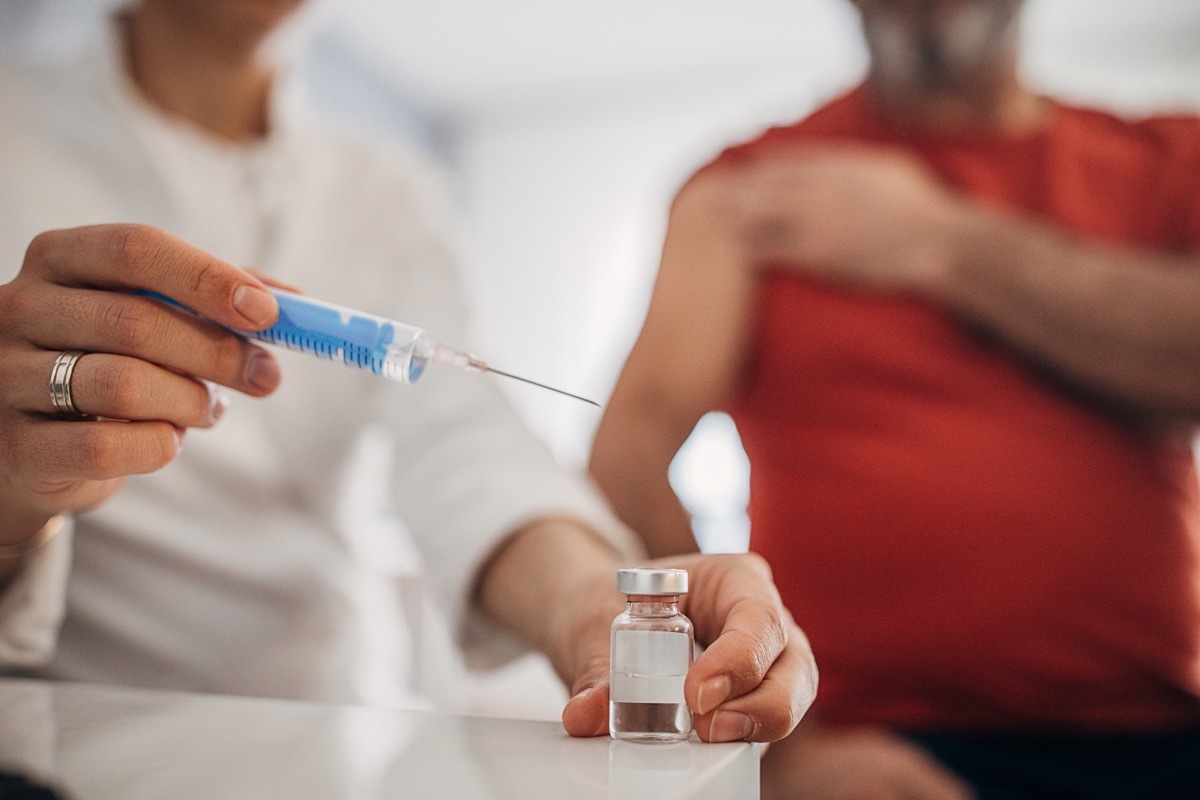
This is not the first time that Innépiex has drawn market medications because ofPotential security issues related to sterility concerns.
While the company had not received any side effects report due to the use of their drugs at the time, on October 10, 2019, the FDA announced that Innovative Pharmaceuticals, Inc. had recalled all sterile aggravated drugs In their expiry period due to "the lack of insurance of sterility" occurring as a result of a routine inspection of the FDA.
RELATED:If you use this medicine, call your doctor now, says FDA.

Surprising side effects of going vegan, expert indicates

