CDC says that only these people should not get vaccine
"If you had a serious allergic reaction (anaphylaxis) or an immediate allergic reaction."
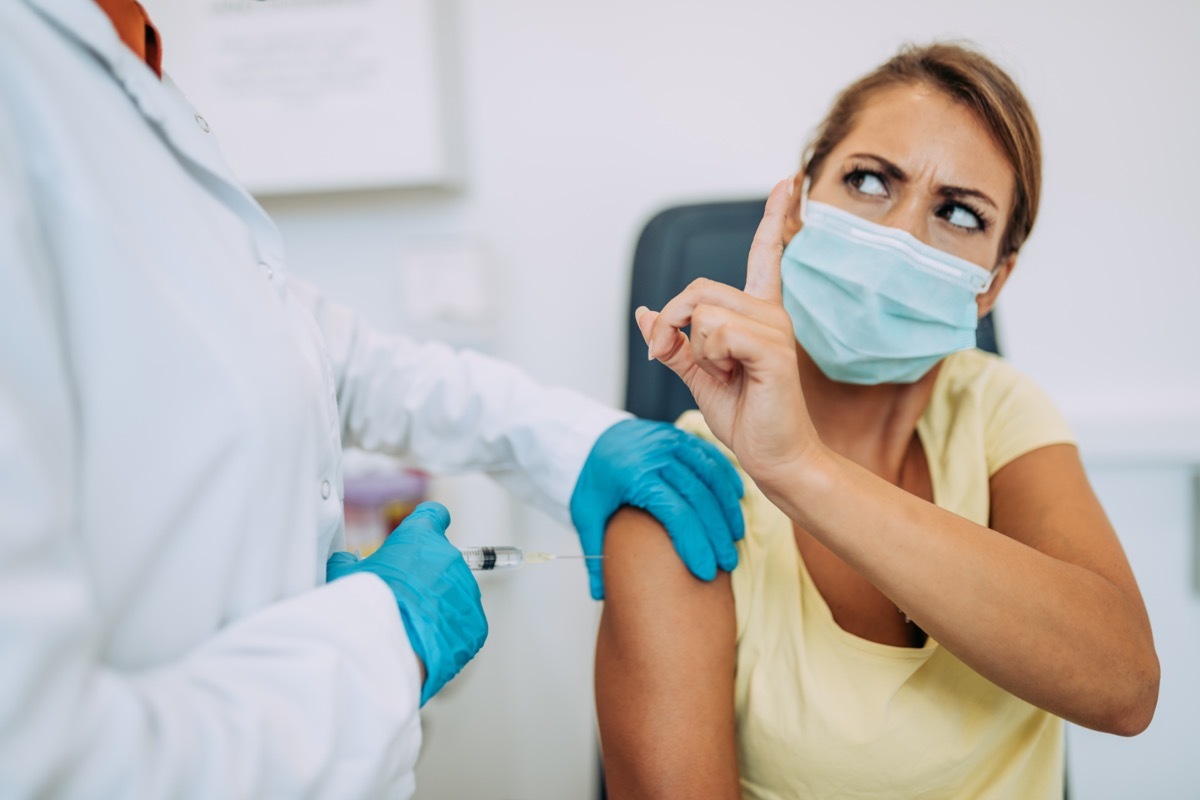
Who can get theirCOVID vaccine? "Everyone 12 years and over is now eligible forGet a COVID-19 vaccination, say itCDC. "Get a Covid-19 vaccine as soon as possible. Generalized vaccination is an essential tool for helping stop the pandemic." So that shouldnot Get their Covid-19 vaccine? It's a very small list. "Covid-19 vaccines can be administered to most people with underlying medical conditions. This information aims to help people in the following groups make an informed decision on receiving a CVIV-19 vaccine." Read on to see who should stay concerned - and to ensure your health and health of others, do not miss theseWithout a sign that you caught Covid and maybe did not know it.
Which should not be vaccinated
Said the CDC: "If you have had a serious allergic reaction (anaphylaxis) or an immediate allergic reaction, even if it did not matter:
- For any ingredient in an Arna Covid-19 vaccine (such as polyethylene glycol), you must not obtain an ARNA COVID-19 ARND vaccine.
- Or after getting the first dose of the vaccine, you should not get a second dose of one of the mRNA.COVID-19 [Feminine Vaccines.
- An allergic reaction is considered serious when a person should be treated with epinephrine or epiplen © or if they have to go to the hospital. To knowCurrent side effects of COVID-19 vaccinesand when to call a doctor.
- An immediate allergic reaction means a reaction within 4 hours of their vaccine, including symptoms such as hives, swelling or respiratory respiration (respiratory distress).
If you are unable to get an Arna Covid-19 vaccine, you can still get a different Covid-19 vaccine type. Learn moreInformation for people with allergies. "
If you have a severe allergic reaction to a Covid-19 vaccine, here's what to do
Said the CDC: "If you had a serious allergic reaction, also called anaphylaxis - after getting the first shot of a Covid-19 vaccine,CDC recommendsthat you do not get a second shot of this vaccine. If the reaction was after an Arna Covid-19 (Pfizer-Biontech or Moderna) vaccine, you should not get a second shot of one of these vaccines. Learn whatCovid-19 vaccines need a second shot.An allergic reaction is considered serious when a person should be treated with epinephrine or epiplen © or if they have to go to the hospital. To knowCurrent side effects of COVID-19 vaccinesand when to call a doctor. "
If you have a non-severe allergic reaction to a Covid-19 vaccine, here's what to do
Said the CDC: "If you had an immediate allergic reaction after having a photo of a Covid-19 vaccine, you do not have to get a second shot of this vaccine, even if your allergic reaction was not serious enough to require Emergency care. If the reaction was after an Arna Covid-19 (Pfizer-Biontech or Moderna) vaccine, you should not get a second shot from any of these vaccines. An immediate allergic reaction occurs in the 4 Hours according to their vaccine and can include symptoms such as hives, swelling and wheezing (respiratory distress). Your doctor can refer you to an allergic and immunology specialist to provide more care or advice.
To knowGet a different vaccine type after an allergic reaction. "
If you get a rash where you had shot, here's what to do
Said the CDC: "CDC has learned the reports that some people have experienced a rash, itchy, swollen or painful where they have shot. These rashes can start a few days more than a week after the first and are sometimes quite big. These rashes are also called "Arm Covid". If you meet "Covid Bram" after getting the first shot, you still need to get the second shot at the recommended interval if the vaccine you need A second shot. Say your vaccination provider you have experienced a rash or "Covid arm" after the first shot. Your vaccination provider can recommend you to get the second shot in the opposite arm. "
If the rash is itchy, you can take an antihistamine. If it is painful, you can take a pain medication such as acetaminophen or non-steroidal anti-inflammatory drug (NSAID).
RELATED: Reason # 1 that you could have cancer, according to science
Backups are in place
Says the CDC: "CDC hasRecommendations provided for COVID-19 vaccination providerson how to prepare the possibility of a severe allergic reaction:
- All people who get a CVIV-19 vaccine should be monitored on site. People who have serious allergic reactions or who have had any type of immediate allergic reaction to a vaccine or injectable therapy must be monitored at least 30 minutes after obtaining the vaccine. All other people must be monitored for at least 15 minutes after obtaining the vaccine.
- Vaccination providers should have appropriate staff, medicines and equipment, such as epinephrine, antihistamines, blood pressure monitor and synchronization devices to check your pulse - at all Covid-vaccination supplier sites. 19.
- If you encounter a severe allergic reaction after getting a Covid-19 vaccine, vaccination providers can provide carefully care and request emergency medical services. You should continue to be monitored in a medical facility for at least several hours.
Learn more about what to expect after being vaccinated for COVID-19, including normal side effects and tips to reduce pain or discomfort. "
CDC monitors serious allergic reaction reports
Said the CDC: "If anyone has a severe allergic reaction after being vaccinated, their vaccination supplier will send a report to theUnwanted event reporting system for the vaccine (VAERS).Vaers is a national system that collects reports from health professionals, vaccine and public manufacturers on adverse events that occur after vaccination. The unexpected adverse events reports appear to occur more often than expected or have unusual schemes are followed by specific studies. "
RELATED: I am a doctor and I warn you that you never take this supplement
People with underlying medical conditions for increased risks of COVID-19
Said the CDC: "Adults of all ages withcertain underlying medical conditionsare at increased risk of serious disease of the virus that causes Covid-19. Covid-19 vaccines are recommended and can be administered to most people with underlying medical conditions.
TheList of high-risk medical conditions that put people at increased risk of serious disease associated with COVID-19is updated regularly when new data is available. "
People who have weakened immune systems, here's what to do
Said the CDC: "People with HIV and weakened immune systems due to other diseases or drugscould be at increased risk for serious COVID-19. They can receive a CIVID-19 vaccine. However, they should be aware of the limited security data:
- COVID-19 vaccine safety information for people who have weakened the immune systems in this group are not yet available
- People living with HIV have been included in clinical trials, although this group's security data is not yet available at the moment.
People with weakened immune systems should also be aware of the potential for reducing immune responses to the vaccine, as well as the need to continue to followCurrent orientationProtect against Covid-19. "
RELATED: Cause No. 1 of obesity, according to science
People who have autoimmune conditions, here's what to do
Said the CDC: "People with autoimmune conditions can receive a CVIV-19 vaccine. However, they must be aware that no data is currently available on the safety of Covid-19 vaccines for people with autoimmune conditions. People in this group were eligible register in some of the clinical trials. You can find more information about the clinical trials of vaccines below. "
People who have already had Guillan Bar Syndrome (GBS), here's what to do
Said the CDC: "People who have already had GBS can receive a CVIV-19 vaccine. To date, no cases of GB has been reported after vaccination among participants in theArna Covid-19 vaccineclinical tests. A case of GBS has been reported in a participant vaccinated in Johnson & Johnson Janssen Covid-19 Covid-19 trial clinical (compared to a case of GBS in those who received placebo). Within a few exceptions, the Independent Advisory Committee on Immunization Practices (ACIP)General Guidelines for Best Practices for ImmunizationDo not include a history of GBS as a precaution for vaccination with other vaccines. "
RELATED: 9 daily habits that could lead to dementia, say experts
People who have already had Bell's paralysis, here's what to do
Said the CDC: "People who have already had Bell's paralysis can receive a Covid-19 vaccine. Bell's paralysis cases have been reported as a result of vaccination among participants in CVVID-19 clinical trials. However, the Food and Drug Administration (FDA) do not judge that these are more than the expected rate in the general population. They have not concluded that these cases have been caused by vaccination. "
After vaccination, follow the current guidelines to prevent the propagation of CVIV-19
Said the CDC: "After being fully vaccinated against Covid-19, you may be able to start doing things that you have stopped doing because of the pandemic. Learn more about what you can doWhen you have been fully vaccinated. "
RELATED:Signs you get one of the "most deadly" cancers
People with underlying medical conditions included in CVVID-19 vaccine clinical trials
Said the CDC: "Vaccine manufacturers report information from clinical trials, including the underlying demographics and medical conditions of people who participated in CVIV-19 vaccine tests. You can find additional information on Covid-19 vaccine clinical trials atclinicaltrials.gov, a funded clinical education database in private and funded by audiences carried out around the world. So you are vaccinated when it becomes at the disposal of you and protecting your life and the lives of others, do not visit any of these35 places you are most likely to catch Covid.

10 healthiest vegetables on the planet to detox and lose extra weight

