The FDA has just published a new alert on this popular drug
The agency says that it has not been approved for specific use.

That you want to relieve the pain of aBuild headache or need to adjust a suddenstomach stomach, your pharmacy cabinet is probably filled to the brim with different medications that you can use. But while the tests and errors have probably helped you to realize thatSome drugs have the ability to relieve a number of symptoms, most are approved for very specific uses. In fact, the Food and Drug Administration of the United States (FDA) has just published a new alert on a popular drug, warning consumers that it is not approved for a particular thing. Read the rest to discover what the agency currently advises.
Read this then:If you use this common medication, the FDA has a new major warning for you.
The FDA is responsible for regulating drugs for consumers.

When it comes to confirming the safety of the things you consume, the FDA leads the charge. The agency is responsible forregulate a wide range Product categories, including prescription and prescription drugs. In terms ofits regulatory requirements, the FDA is supposed to approve drugs to protect itself against the sale of poorly elastic or potentially harmful drugs, as well as to supervise the recall of any medication and warn the public of the potential adverse events associated with them.AE0FCC31AE342FD3A1346EBB1F342FCB
"Americans receive up to 3 billion pharmaceutical products each year, and millions of people receive medical devices such as hip and knee implants. All medicines and medical devices include inherent risks, but it is FDA's obligation to deal with the serious risks that can be avoided and has managed: "explains the agency.
Now the FDA has published a new alert on the use of a popular drug.
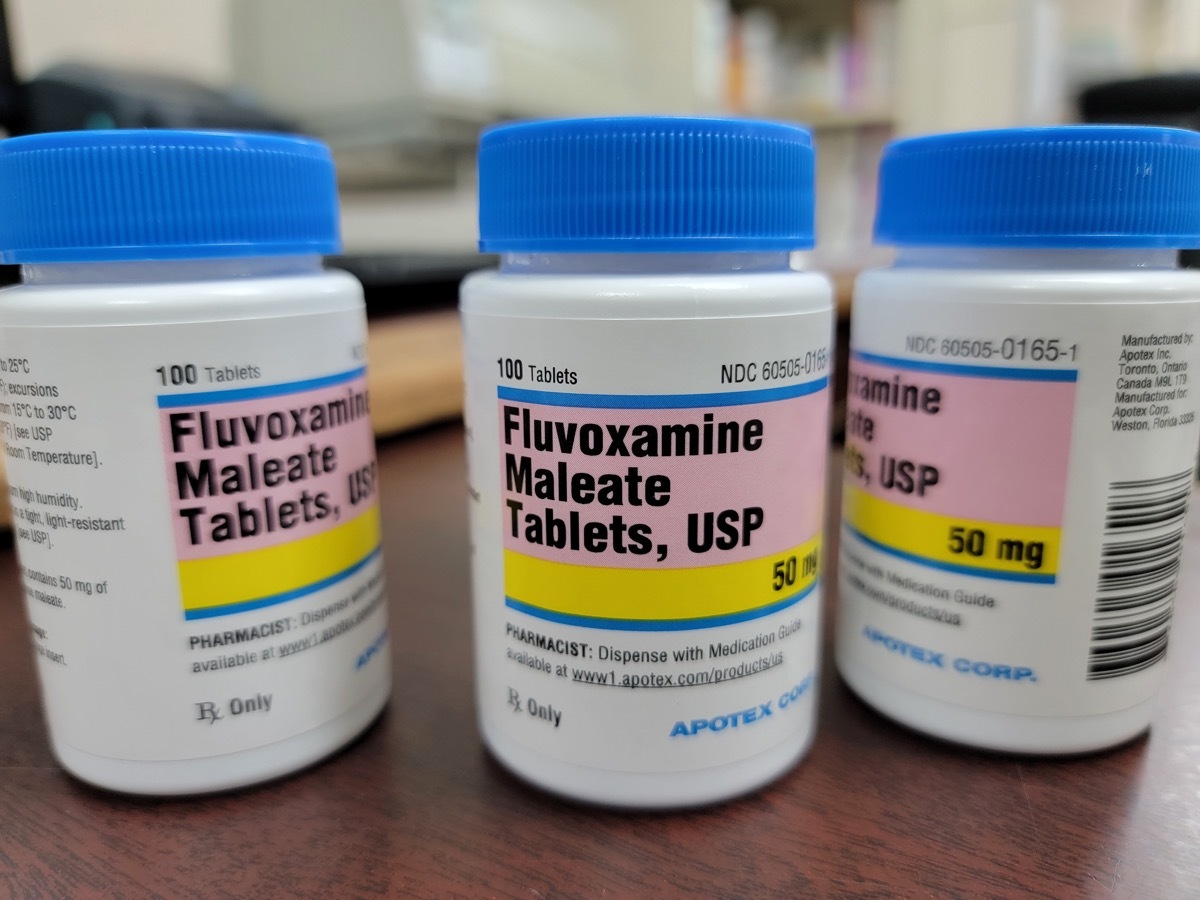
On May 16, the FDA announced that itnot to allow Fluvoxamine medicine as a covid treatment. According to the alert, a group of health care providers led byDavid R. Boulware, MD, professor of medicine at the University of Minnesota, submitted a proposal in December 2021, requesting that the agency approves this drug for the "ambulatory treatment of adults aged 24 and over with the results of positive tests of SARS- Viral Test VOC -2 to prevent progression to a severe cocovone - 19 and / or hospitalization. "
The group included information from clinical trials studying the use of fluvoxamine for COVID treatment in their proposal. But after a scientific examination staff of the FDA has evaluated the information available, the agency revealed in a 27 -page alert which chose not to issue the requested emergency authorization (EUA).
RELATED:For more up-to-date information, register for our daily newsletter.
The agency says that there is not enough data to authorize the drug to use against COVVID.

Fluvoxamine is a selective generic inhibitor of serotonin reuptake (ISRS) which ismost commonly used Treat obsessive compulsive disorder (OCD), according to Mayo Clinic. The FDA approved the use of drugs for OCD, as well asin the treatment depression, anxiety and other mood disorders. But in its new alert, the agency said that the data provided did not prove that this drug is effective in the processing of infections caused by the coronavirus.
"On the basis of examining the scientific evidence available, the FDA determined that the data is insufficient to conclude that fluvoxamine can be effective in the treatment of non-hospitalized patients with COVID-19 to prevent progression to a serious illness and / or hospitalization ", the FDA wrote. According to the agency, there were "uncertainties" on the results of the main clinical trial cited, "design limitations" with the studies of real world data, and the benefit of processing this medication for a severe covid 'was "not convincing".
Not everyone is satisfied with the FDA decision.

Boulware, who led the thrust for the authorization of fluvoxamine in the treatment of Covid, tweeted on May 16 that he was "disappointed" from the FDA decisionrefuse his proposal . The agency has already given the green light to other treated treatments, including Paxlovid Pill of Pfizer and the Molnupiravir medication of Merck. But according to Boulware, there is still value in the FDA authorizing other popular drugs such as fluvoxamine for the treatment of coronavirus infections.
"There are effective therapies that are available. But but Not everyone has access for them. Not everyone can tolerate them. Some people have contraindications, "he told Reuters." And if you go elsewhere in the world, low and average income countries, they do not have access to any therapeutics. ""
Read this then: If you drink this popular drink, the FDA has a new major warning for you .

If your Android battery suddenly flows, it might be why

