If you use this common medication, talk to your doctor now, the FDA warns
Certain Pfizer drugs have been recalled after recent FDA tests.

Whatever in your medication office, you want to have the confidence that everything you take is sure to consume. Whether you use a daily prescription or a range for antacids after a large meal, we rely on drugs to help you rather than injure ourselves. Now, acommon drug has just been recalled and you will want to reach out to your doctor immediately if you have one of the touched bottles. Read it to find out more about the PFIZER product than the administration of American foods and drugs (FDA) (FDA) could do more harm than good.
RELATED:These heartburn and pain drugs have been recalled, the FDA warns.
The name of Pfizer is now easily recognized.

In recent years, Pfizer has become a household name. The company is recognized for making the country's first COVVI-19 vaccine, alongside Biontech, having been originally approved by the FDA in 202020. According to the annual revision of Pfizer's 2021, almost3 billion doses The vaccine was produced in 2021 and 4 billion are planned to be manufactured in 2022.AE0FCC31AE342FD3A1346EBB1F342FCB
Before making titles during the pandemic, however, Pfizer was widely known for product treatments forOther medical conditions, from the depression (in the form of Pristiq) to fibromyalgia (via Lyrica). Now, the pharmaceutical company recalls a selection of many popular drugs, which is used to treat an even more common condition.
Five pieces of Pfizer have been recalled.
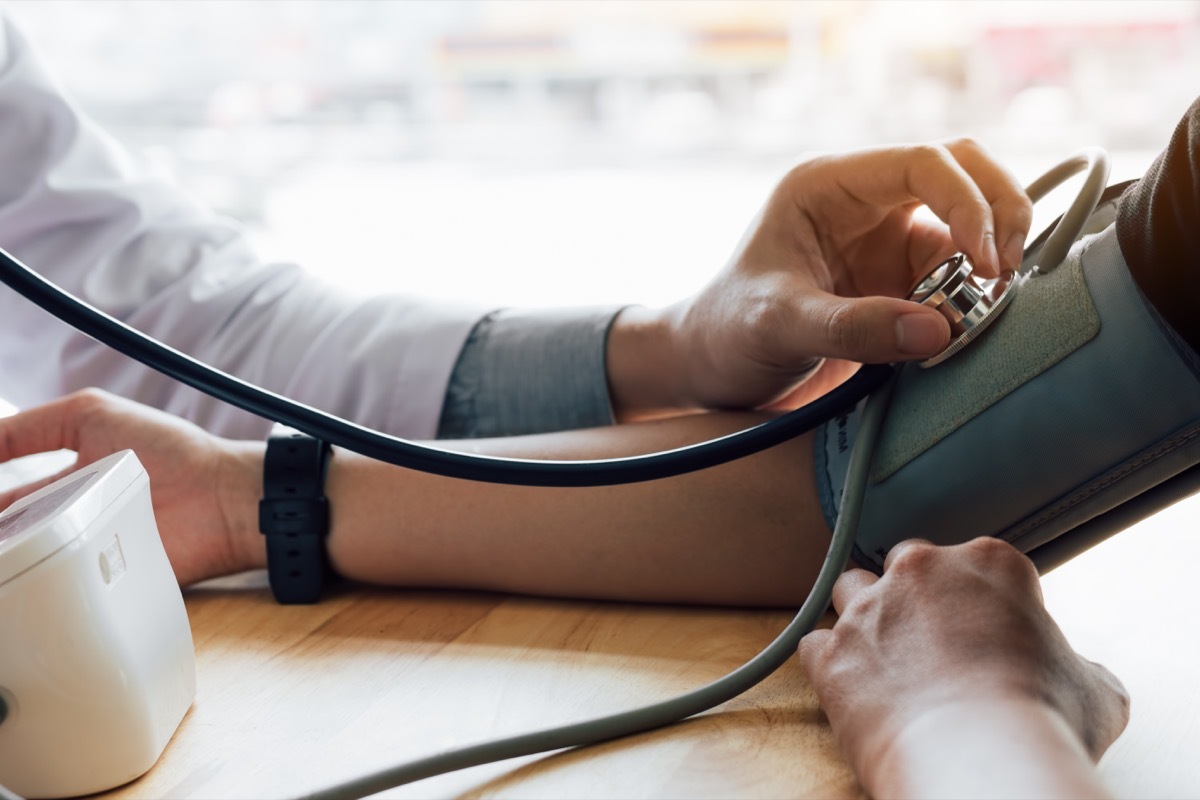
Disease control and prevention centers (CDC) believe that almostHalf of the Americans have hypertensionBut only about a quarter of these people have their condition under control. Accupril is a drug often prescribed for the treatment of high blood pressure, but some of these products could now pose greater threats to health.
On April 22, the FDA announced that Pfizer wasRemember voluntarily Five of many accupgrine tablets after tests have found high levels of nitrosamine, or nnitroso-quinapril, a potential cancer-catering agent. According to the announcement of the FDA, nitrosamines are often found in water and food, as well as guilled and grilled meats, dairy products and vegetables. The risk of cancer is increased when people are exposed to higher levels over prolonged time.
RELATED:For more information up to date, sign up for our daily newsletter.
You should check your medication to see if it's part of the recall.

In addition to the treatment of hypertension, you can also take the battery as a complementary therapy for the management of heart failure, when associated with diuretics and / or digitization (conventional therapy approaches), According to the FDA warning. People who take an battery (also known by its generic name, Quinapril hydrochloride) so that all these conditions should check whether their products are part of the recall.
On your bottle, search for the national medication code (NDC), indicated on the top of the label, as well as the Lot number date and the expiration date, indicated on the left side of the label. All the products concerned each had 90 tablets.
The product recalled of 10 mg has an NDC of 0071-053-23, a number of lots of DR9639 and an expiration date of March 31, 2023. Recalled the products of 20 mg have an NDC of 0071-0532-23, number DX8682 and DG1188 lot numbers and expiration dates of March 31, 2023 and May 31, 2022, respectively. The products recalled 40 mg have an NDC of 0071-0535-23. Products in the number of DX6031 lots have an expiration date of March 31, 2023, while products in the number of CK6260 lots have an expiration date of May 31, 2022.
The prizes of products recalled were distributed on wholesalers and distributors across the United States and Puerto Rico between December 2019 and 2022, the FDA said.
If you have one of the products concerned, contact your health care provider.

In the announcement of the FDA, Pfizer said that the profile of accupgil benefits / risks "remains positive on the basis of the data currently available" and there has been no report of adverse events related to the recall to this day. And while long -term ingestion of nitrosamines can be associated with an increase in the risk of cancer ", there is no immediate risk for patients taking this medication."
However, if you have this medication recalled, you are advised to contact your doctors or health care providers on alternative processing options. For more information on the return of the product and reimbursement, patients must also contact SEDGWICK at 888-345-0481 between 8 a.m. and 5 p. Oriental time from Monday to Friday. The FDA also asks you to report undesirable effects or quality problems to the MEDWATCH unwanted event report Online or mail or ordinary fax program.
Wholesalers and distributions are invited to immediately stop distribution and in quarantine the affected products they can have. If the products were distributed more to additional locations, companies should inform recipients and "perform a sub-re-chief".
RELATED: If you use this common medication, call your doctor now, the FDA warns .

4 reasons why Rose should have stayed with Cal in "Titanic"

