All you need to know about the new coronavirus treatment
Dr. Anthony Fauci is Lauding Vedesivir, saying that he proves "that a medicine can block this virus".
There was a great shortage of new and encouraging surrounding the outbreak of coronavirus. But newly released results of clinical trials RemDesivir could simply be the good news that we wanted.REMDESIVIR is an anti-viral drug Produced by American Biotech Company Gilead, andAnthony FauciMD, epidemiologist at the head of the working group on the coronavirus of the White House, said RembDesevir "proved ... that a drug can block the virus." Here's what we know about REMDESIVIR far. And for more cures thatnotjob,These are fake healings Covid-19 you should ignore right now.
1 Dr. Anthony Fauci expressed a remarkable optimism about RemDesivir.
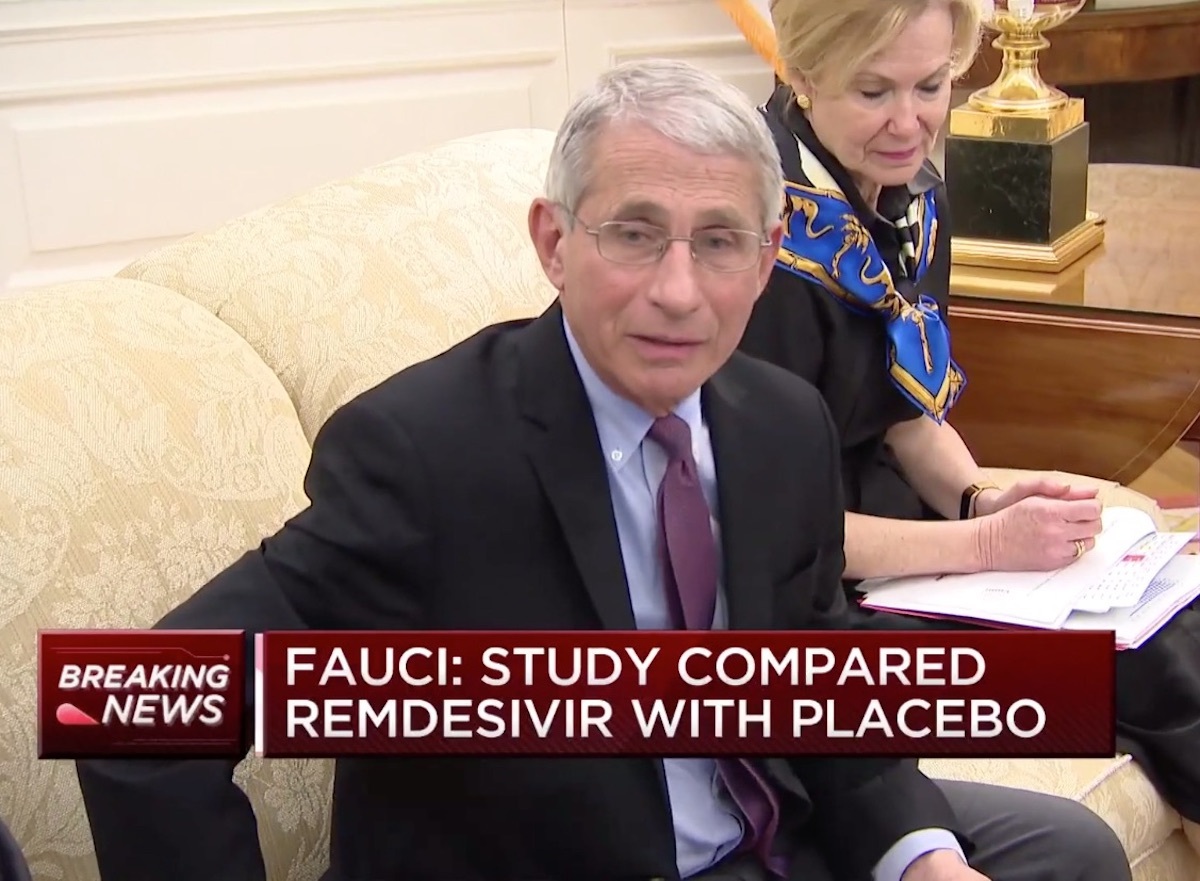
In a press briefing on April 29, Fauci highlightedThe effectiveness of REMDESIVIR In dealing with those coronavirus, calling it "very good news". In a study of the National Institutes of Health, the mortality rate was 8% for the group receiving REMDESIVIR compared to 11.6% for the placebo group. Fauci revealed that "the data show that RemDesivir clearly has a positive effect and significant cut in reducing the recovery time."
"It's really important for a number of reasons," he added. "What he has proven is that a drug can block the virus. ... It's very optimistic."
2 The FDA is set to allow emergency use of REMDESIVIR.

According toThe New York Times, The Food and Drug Administration (FDA) intends to announce "on Wednesday" thatREMDESIVIR is authorized for emergency use Treating patients Covid-19, citing a senior executive as the source.
3 RemDesivir is produced by an established biotechnology company Gilead.

REMDESIVIR was developed by Gilead, a US based biotechnology in 1987 specializing in the research, development and commercialization of anti-viral drugs. The company successfullyDevelopment of effective treatments used to control or maintain the health of those with HIV, hepatitis B, hepatitis C and influenza.
4 Clinical trials of remdesivir began in February.

In an open letter on the company's web site on 10 April, Gilead CEODaniel O'Day wrote: "It has been only two months since the first clinical trials began Since it can take a year or more for the first clinical data for treatment of inquiry, it is remarkable that we expected. having toFirst REMDESIVIR test data so early."
5 And there are seven ongoing trials remdesivir to accelerate testing.

O'DDA added that "seven clinical trials have been initiated to determine whether REMDESIVIR is a safe and effective treatment for Covid-19."
In a trial at the Medical Center of the University of Chicago, 125 people Covid-19 were treated with RemDesivir, of which 113 were serious.Kathleen Mullane, Do, a specialist in infectious disease at the University of Chicagosupervise REMDESIVIR studies To the hospital, told Statnews "Most of our patients have been dischargedThat's great. We had two patients perished. "
But shortly after, thereafter, according to an exclusive reportThe financial times,REMDESIVIR deployed in the first randomized clinical trial"Scientists and disappointing investors who had high hopes for Rembesivir."
6 RemDesivir was initially created to treat Ebola.
Gilead initially established as a treatment for REMDesivirWest African outbreak of Ebola. But clinical trials have shown that RemDesivir was not as effective as other drugs, and it was eventually abandoned as Ebola therapy. And for other pandemics, checkHow is the coronavirus stacked relative to other pandemics?
7 REMDESIVIR is still in an experimental stage and not approved worldwide for use.

As news of RemDesivir are very encouraging, it is still early intraditionally complicated process. In his open letter, O'Day wrote: "Remdesivir is an investigational therapy and has not been approved for use anywhere in the world in the broader effort to determine if it is from. a safe and effective treatment, we have a way to go. "and for more answers to your FAQ Covid-19, check13 Current Questions of Coronavirus - Answered by Experts .

If you bought this from Costco, stop it immediately

23 effects of divorce that people do not speak, according to experts
