FDA just revoked Authorization of this coronavirus popular treatment
After making the cuffs for months, these drugs will no longer be used on patients who fight against COVID-19.
The medical community worked tirelessly to findEffective treatment for COVID-19 since the first days of the pandemic. And while we have acquired a better understanding of how the virus spreads and how we can help combat outbreaks, finding a medicine that can be used for patients with treating disease is always a battle in progress. Unfortunately, Monday marked another ignition failure in searching for a possible solution:The Food and Drug Administration (FDA) announced that it had revoked its prior authorization for the use of hydroxychloroquine and chloroquine for the treatment of coronavirus.
Drugs, which are commonly used to treat malaria and lupus, has made the headlines after first showing signs of hope in clinical trials as an effective therapy for the new coronavirus.Hydroxychloroquine was pushed further into the spotlight After being presented as a solution by the PresidentDonald Trump, Who claimed to be taking the medicine.
At the end of March, the FDA issuedAuthorization for emergency use of hydroxychloroquine (HCQ) and chloroquine (CQ) for COVID-19. Then, at the end of April, the FDA issued the following warning about the drugs:
FDA fears that hydroxychloroquine and chloroquine are used inappropriately to treat non-hospitalized patients for coronavirus disease (COVID-19) or to prevent this disease. We have authorized temporary use in hospitalized patients with COVID-19 in clinical trials are not available, or participation is not possible, through an emergency authoritency (AU) . These drugs have a number of side effects, including serious heartbeat problems that can be life in danger.
In addition to being ineffective in the treatment of coronavirus, hydroxychloroquine and chloroquine have also been linked toCardiac complications and other serious side effects In coronavirus patients they have been used on. But it was not until the FDA took a tighter review of the evidence and the absence of sufficient data for recent studies that it reversed its ECU.
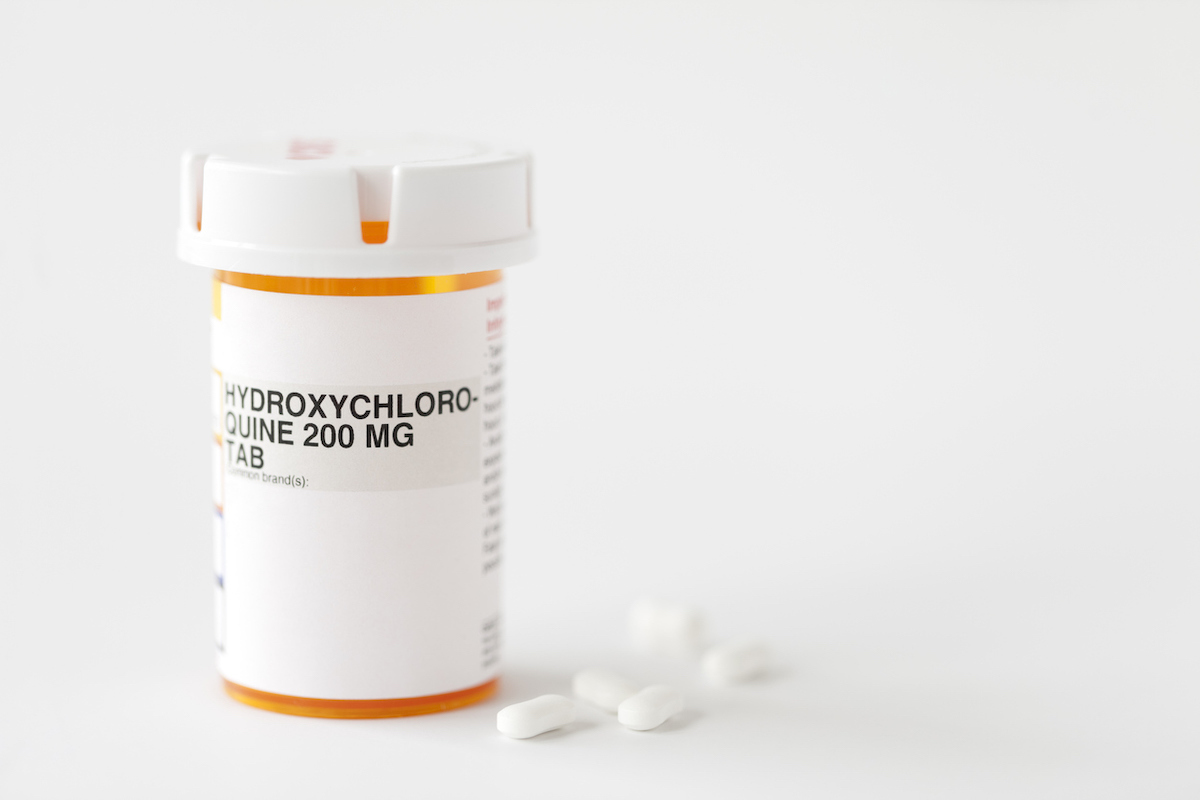
"It's no longer reasonable to believe thatOral formulations of HCQ and CQ Can be effective in CIVID-19 treatment, nor is it reasonable to believe that the known and potential benefits of these products outweigh the known and potential risks, "Denise Hinton, Scientific Director of the FDA, wrote in a letter to the authority of advanced biomedical research and development (BARDA). She added that "we also knew it was important to provide helpStable supplies of drugs For patients with lupus and rheumatoid arthritis given the increased demand ".
RELATED:For more information up to date, sign up for our daily newsletter.
But the public saga of these pharmaceuticals is not yet: WHO still leads tests and internal data review to finally determine the effectiveness of drugs. They say they are waiting to make a decision aboutHydroxychloroquine and Covid-19 in mid-June. And for more on the treatment of new coronaviruses, seeCovid-19 Survivor saved by this hallucinating, first procedure.

Eating 1.5 teaspoon of this everyday life increases your heart health, a new study says

