The new FDA approved COVID treatment works only for these people
This useful treatment can actually be harmful for some cases of coronaviruses.

Another step forward in the fight against coronavirus was approved by the US Food and Drug Administration (FDA) for emergency use authorization. February 9, Eli Lilly-Bamlanivimab and Etesevimab Antibody Treatment Administered Togetherreceived approval. The treatment can be essential to help people with COVID recover quickly. However, it's not for everyone. Continue reading to learn the uses and limits of this coronavirus treatment and make sure you stay healthy,If you have more than 65 years, you can miss this symptom of Covid..
The new CVID treatment approved by the FDA is recommended only for some people.
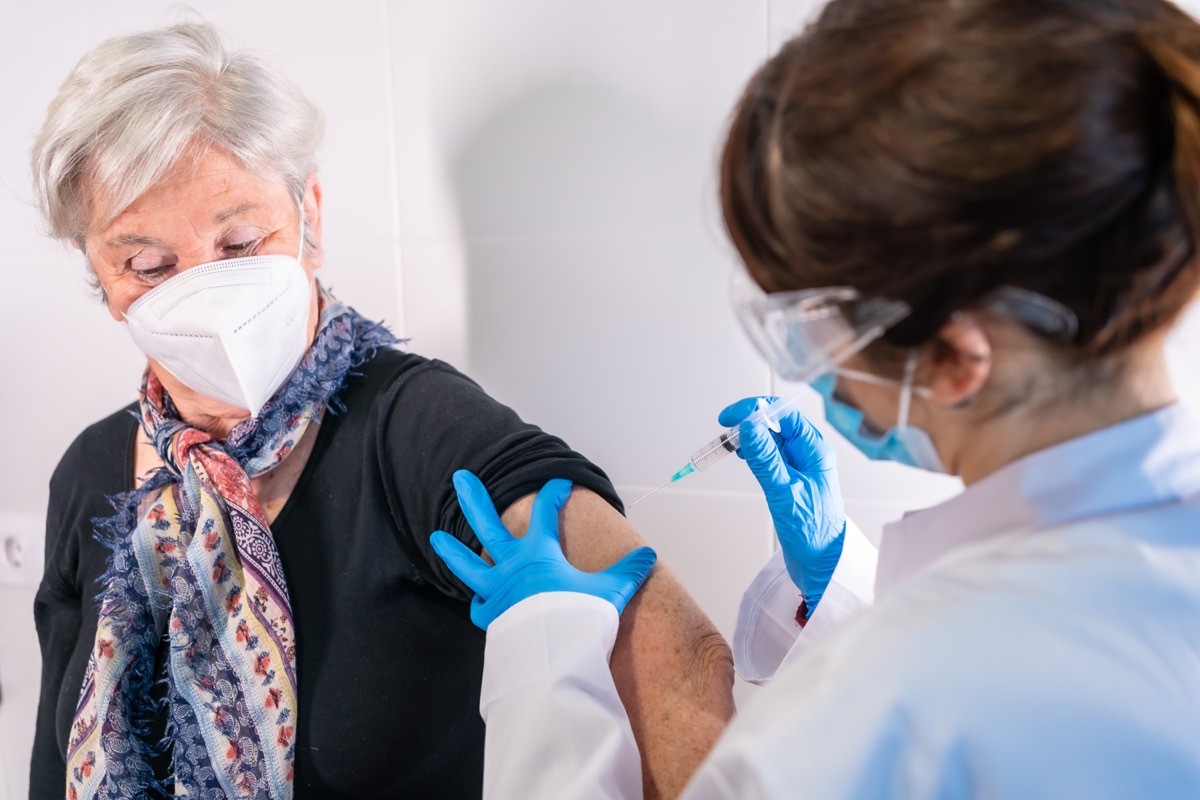
In order to receive this newly approved treatment, you must enter one of the groups exposed by Eli Lilly and the FDA. People who test positive for COVID and haveLight symptoms to moderate or are at high risk of progressing in severe covidation or severe hospitalization to this treatment. In addition, the FDA noted that people can only receive treatment if they are 12 years old or older and weigh at least 88 pounds. And for more information up to date,Sign up for our daily newsletter.
People already at the hospital are not eligible for treatment.
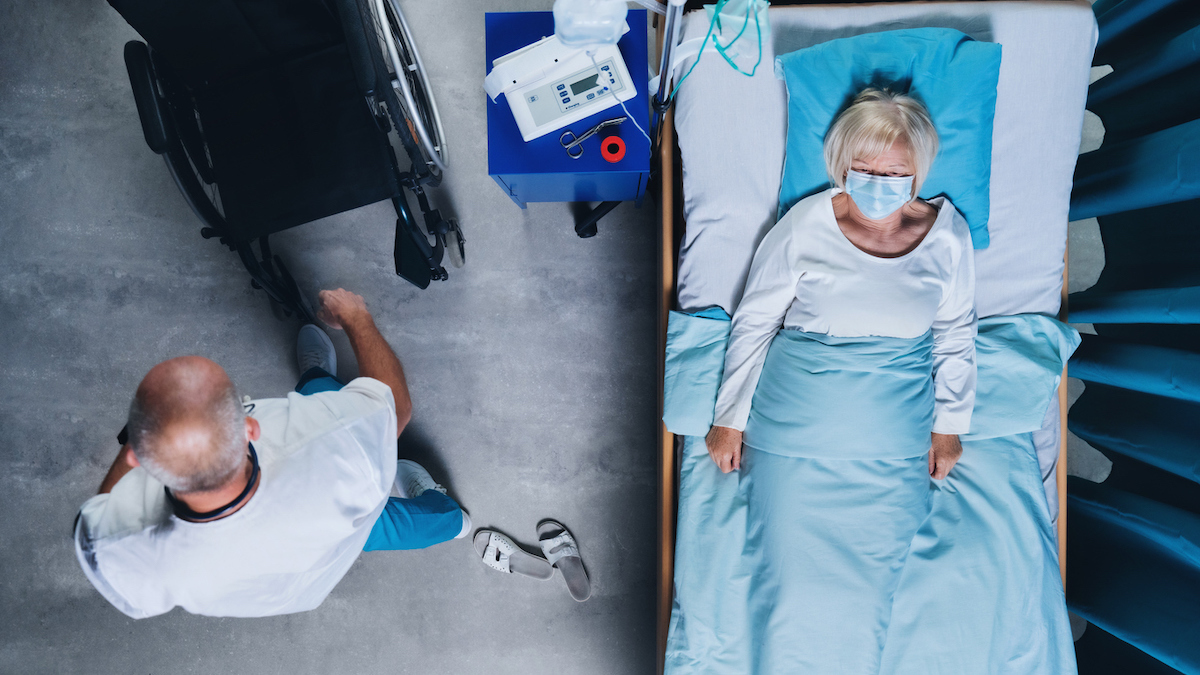
While the treatment is suggested for preventive use for a patient who could go to agrave Covid and hospitalization, the FDA explicitly notes that it is not used for people who are already in the hospital. "Bamlanivimab and Etesevimab are not allowed for hospitalized patients due to COVID-19 or oxygen therapy due to Covid," says FDA.
The treatment has not been studied on hospitalized cavidant patients. Through the FDA, "monoclonal antibodies, such as Bamlanivimab and Etesevimab, may be associated with worse clinical outcomes when administered to hospitalized patients with COVID-19 requiring high flow oxygen or mechanical ventilation". And for more ways to avoid a serious coronavirus affair,This common medicine could save you from the gravity of COVID, a new study says.
Treatment reduces the risk of 70% COVID hospitalization and deaths.
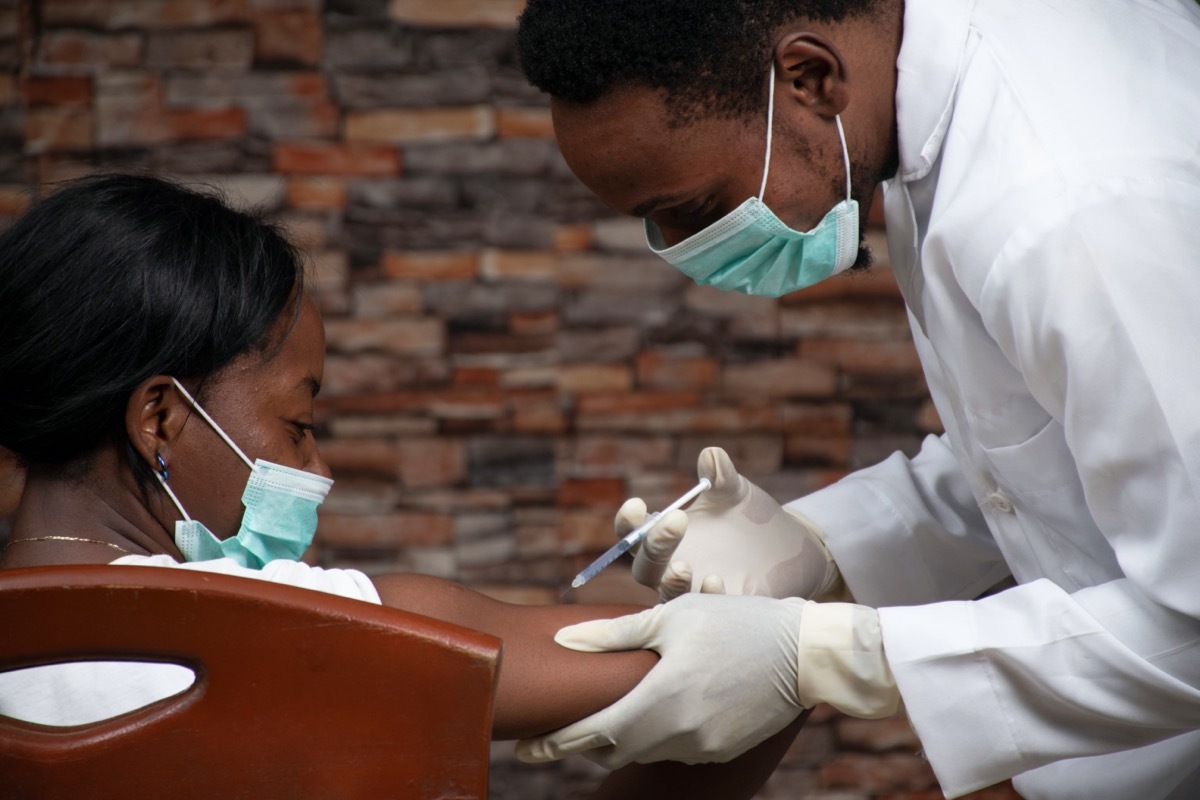
Eli Lilly reported that a trial trial in January showed that their combination treatmenthelped to reduce the risk hospitalizations and deaths related to Covid by 70%.
The treatment will be available immediately. According to Eli Lilly, there are already 100,000 doses ready from and 150,000 additional doses to come throughout the first trimester. The company plans to manufacture up to one million doses by mid-2021. And for more your coronavirus risk,If you did that, you are twice as likely to develop severe covidation.
And it's just a shot.
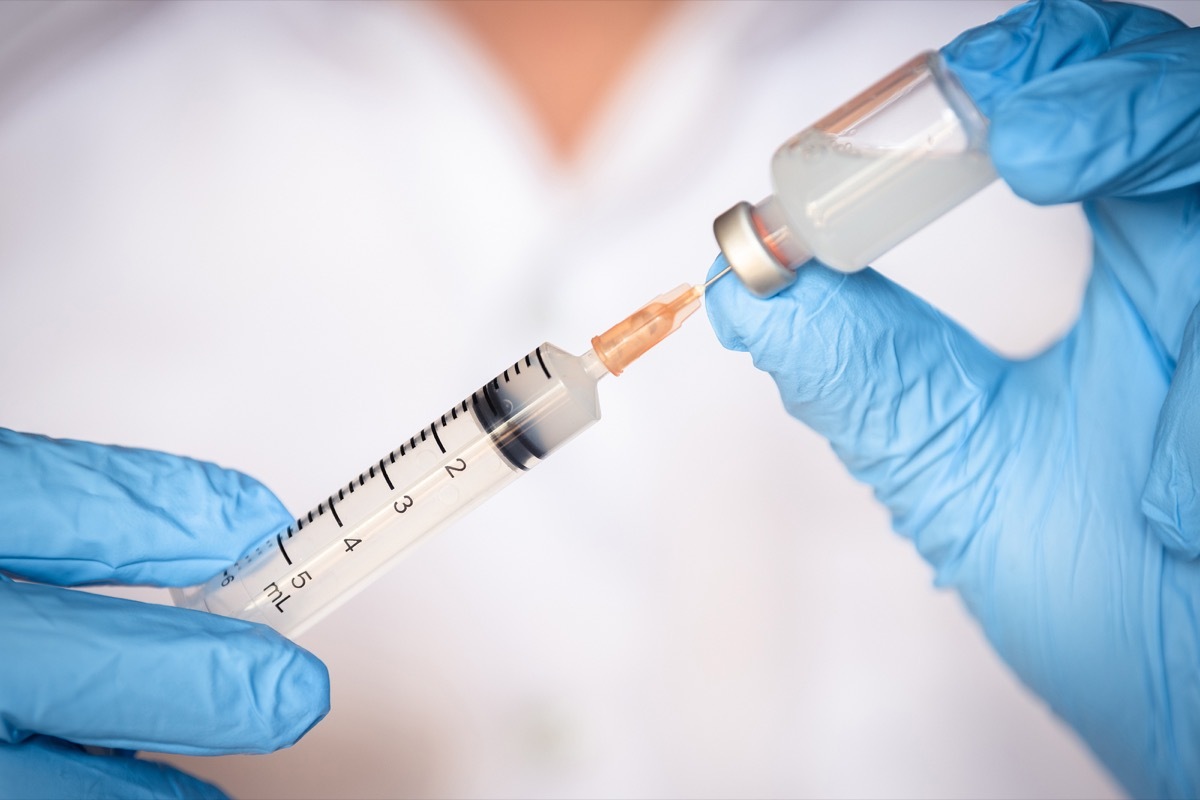
The treatment is administered within one shot which is a combination of Bamlanivimab and Etesevimab. The FDA reports that when administered, it significantly reduces hospitalizations and deaths related to COVID within 29 days, compared to placebo.
The treatment uses monoclonal antibodies, which are laboratory proteins that mimic the ability of the immune system to combat harmful pathogens such as viruses. "Bamlanivimab and Etesevimab are specifically targeted to work against the Spike protein in COVID by blocking the ability of the virus to attach and enter human cells. And for more ways to stay healthy,These 3 vitamins could save you discoveries from the severe Covid study..

Wendy's stops serving hamburgers in some places due to the shortage of meat

