This vaccine has been suspended in Canada for people under 55 years old.
The decision to terminate JABs in younger people stems from a rare blood coagulation reaction.
Covid-19 vaccines currently available around the world come with the potential ofNon-serious side effects Like a painful arm, fever and chills that usually fall within 48 hours of shooting. But health leaders in some countries have recently become alarmed by a vaccine, in particular, can very rarely a serious response in some people, which has led some governments to retain doses. Canada has become the last country to do it,Suspension of the use of the AstaZeneca Covid vaccine For people under 55 years old. Read to see why the shots have been arrested and for more things about how the oldest population can prepare for their doses, discoverIf you have more than 65 years, the CDC says it expects this after your COVID vaccine..
Canada has stopped AstraZeneca doses in people under 55 due to concerns about blood clots.
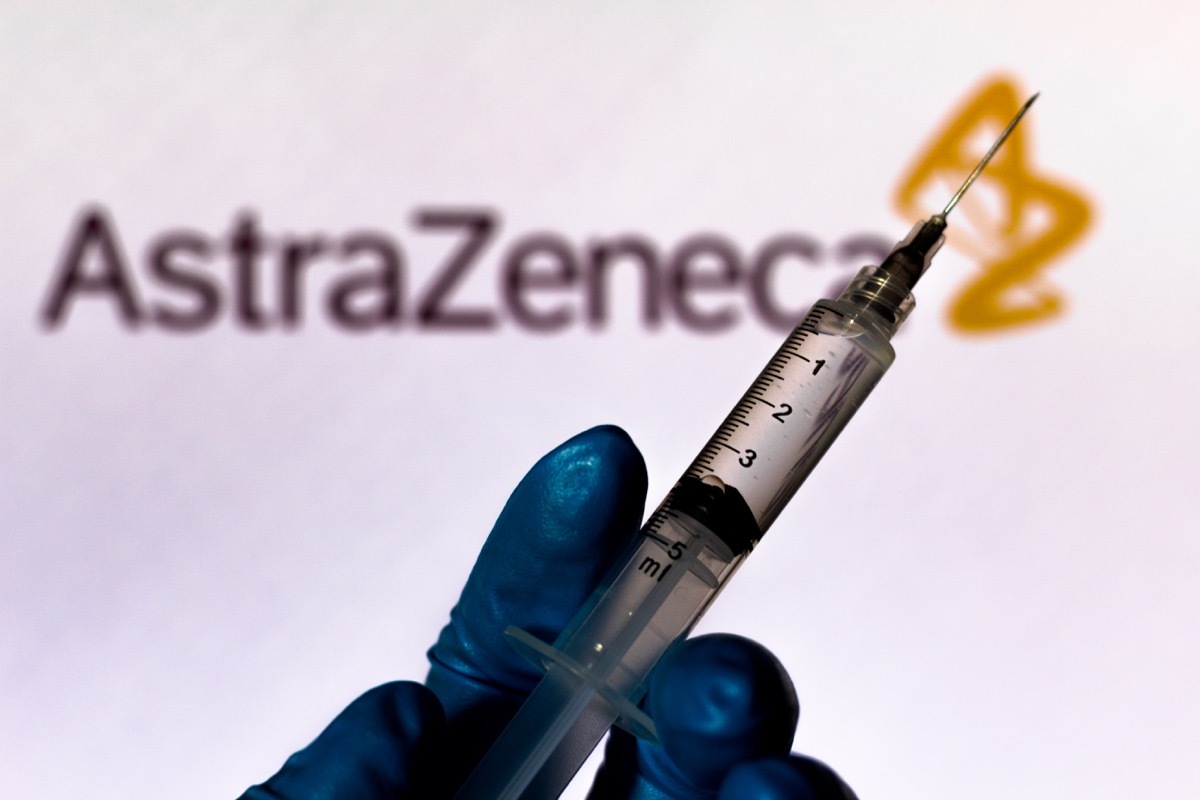
On March 29, the National Consultative Committee of Canada on vaccination announced that it had decided toTemporarily stop the AstaZeneca vaccine administration With those under 55 years after rare cases of blood clots - medically known as the protathistic immune thrombocytopenia induced by the vaccine (VIPIT), in some European countries. Most patients who suffered the reaction threatening by life at AstraZeneca vaccine were women under 55 years old.
"After VIPIC-based analyzes assessing the risk of COVID-19 sick by age and considering that alternative products are available (ie mRNA vaccines), of what It is known at that time, there is a substantial uncertainty about the provision of AstraZeneca Covid-19 vaccine to adults under the age of 55 as the potential risks associated with VIPIT, particularly at rates. Lower estimated, "said the committee officials in a statement.
And for more vaccine responses to search,If 1 of these 3 body parts begin to swell after your vaccine, call a doctor.
Health officials have made their decision on the basis of new European data that have changed risk levels.
Canadian officials said that their decision came after new data from Europe showed that the risk of reaction is 1 out of 100,000, which is much higher than that of 1,000,000 previously before,The Guardian reports. The data also indicate theMortality rate for affected persons was as high as 40%.
According toJoss Reimer, MD, Member of the Working Group on the Implementation of the Canadian Province of Manitoba, theRare coagulation reaction Generally develops four to 20 days after receipt of the dose and has symptoms similar to a stroke or cardiac accident. "Although we still believe that the benefits for all ages outweigh the risks I'm not comfortable with" probably ". I want to see more data out of Europe so I know exactly what That this risk-benefit analysis is, "she said. And for more information on when you need a call back, checkThe CEO of Moderna says it's how many times you will need a Covid vaccine.
Only seven cases of blood blots on several million patients have been reported.
The AstraZeneca vaccine has seen a rocker deployment that most others due to supply problems andQuestions about its effectiveness Following inconsistencies in the clinical data provided by the Company. But the health agency of the European Union has released the vaccine on March 19, the appellant "safe and effective"And it is unlikely to increase the risk of blood clots.
Nevertheless, Canadian health managers have defended their decision toKeep using the vaccine in younger people. "It is reasonable to pause for a period of time as this [risk] continues to be evaluated"SUPRIYA SHARMA, MD, Senior Medical Advisor to Health Canada, said. "I understand it well this can be confusing. Especially for this vaccine, which has had a lot of confusion that surrounds it."
And for more Covid news sent directly to your inbox,Sign up for our daily newsletter.
The deployment of vaccines in Canada should not be significantly delayed by the decision.

Canadian health officials also stated that the suspension would probably not delay the total immunization efforts.AstraZeneca doses are a smaller percentage Vaccines available for use in the country, CNN reports. However, Canada is still planned to receive 1.5 million doses of the United States AstraZeneca vaccine this week, as part of a case affected by the PresidentJoe Biden share portions of the national stock. To date, the AstraZeneca vaccine has not been approved for use in the United States and for more information on how to prepare your gunshots, checkDo not do this at night before your vaccine appointment, say experts.

Steven Spielberg admits that the emblematic film had a negative impact: "I really regret it."

