If you have this vaccine reaction, the CDC says try Johnson & Johnson
The Agency makes new clinical considerations now that a third Covid vaccine is available.
When it comes toGet the coronavirus vaccine, health officials recommend that you do not exchange vaccines because they can not yet be sure howEfficient and safe mix two different vaccines would be. This means that you should not go to your second vaccine appointment and try to ask Pfizer when you got the Moderna vaccine for your first dose. However, disease control and prevention centers (CDC) allowed some flexibility in some cases. If you have a particular vaccine reaction at the first dose of Moderna or Pfizer vaccines, the CDC now says that you can try Johnson & Johnson as a second dose. Read on to find out why you might need to change vaccines and for more tips on vaccines,If you take this common medicine, talk to a doctor before your vaccine..
If you had an allergic reaction to Moderna or Pfizer vaccines, you can try Johnson & Johnson vaccine.

If you have aAllergic reaction to the first dose of modernization vaccines or pfizer, then vaccination providers can use Johnson & Johnson (or Janssen) vaccine as a substitute for your second dose, depending onJessica MacNeil, MPH, epidemiologist at the National CDC Center for Vaccination and Respiratory Diseases.
"In exceptional situations where the first dose of an Arna Covid-19 vaccine was received, but the patient is unable to complete the series with the same IRNA Covid-19 vaccine, for example due to a contraindication. , a single dose The Janssen Covid-19 vaccine can be administered at a minimum interval of 28 days from the 19-day vaccine dose Arma Covid-19, "said MacNeil at an emergency meeting. March 1 of the CCC Vaccination Practices Advisory Committee, as indicated by CNBC. She added that this should only be done "under the supervision of a health care provider", such as safety and efficiency to obtain a painting of Moderna or Pfizer and one of Johnson & Johnson has not yet been tested. And for more than the CDC on vaccine safety,The CDC does not tell this within 2 weeks of your Covid vaccine.
You should not get a second dose of Moderna or Pfizer if you have some allergic reactions to the first.
This new practice could be useful for people who have an allergic reaction to the first dose of Moderna or Pfizer and can not continue their immunization process - which means that they are not "fully vaccinated" against Covid. Currently, the CDC tells you "should not get the second dose"Moderna or Pfizer vaccines If you have experienced a serious or immediate allergic reaction to the first dose. According to the CDC, an allergic reaction is" considered serious when a person has to be treated with epinephrine or a spinjet or if They must go to the hospital. "An immediate allergic reaction occurs within four hours of vaccination and should not necessarily be considered serious. And for more extreme vaccine reactions,If this happens after your vaccine, the FDA says you should call 911.
Serious allergic reactions are rare with Covid vaccine.

Although this guideline change can help some, the vast majority of people will not need to consider it. After all, the CDC states that severe allergic reactions, also called anaphylaxis, can occur with any vaccine but areRare after the coronavirus vaccine. In fact, they report that only about two to five people per million people vaccinated in the United States had anaphylaxis after getting the Covid vaccine. "This type of allergic reaction occurs almost always within 30 minutes after vaccination," said CDC. "Fortunately, vaccination providers have medications available to effectively treat patients who undergo anaphylaxis after vaccination". And for more information up to date,Sign up for our daily newsletter.
The CDC says they change guidelines to include Johnson & Johnson vaccine.
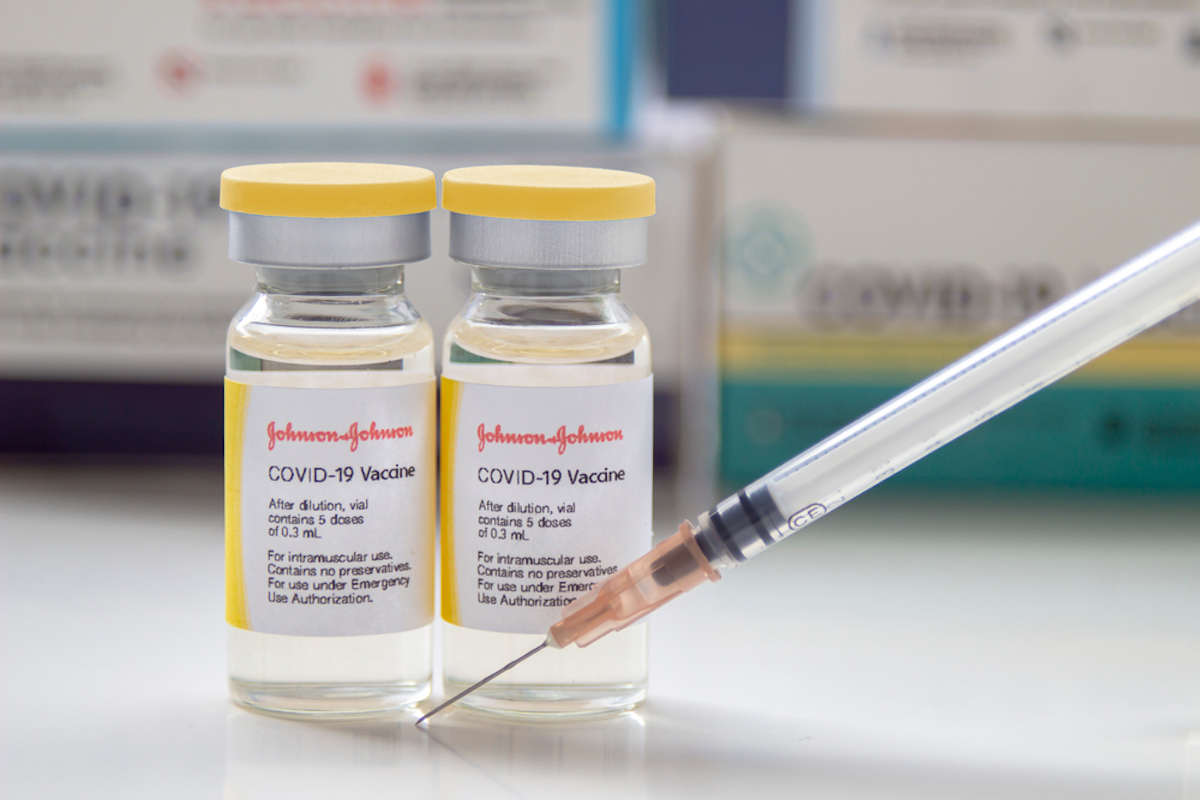
The CDC retains up-to-date Americans with the best vaccine practices for Moderna and Pfizer thanks to their clinical consideration guidelines. Given that Johnson & Johnson, the vaccine was just authorized recently, this vaccine is not yet included in these guidelines. According to a document published by the CDC on March 1, the agency "Clinical considerations are being updated"To include this new vaccine. Given how much vaccine at a shot is different from Moderna and Pfizer, it is likely that a substantial number of new updates and new recommendations from the CDC. And for more information on this vaccine,These are the side effects of the new Johnson & Johnson vaccine, said FDA.

These were the first states to mandate masks. Here's how they do.

