Trump takes this experimental coronavirus treatment
Regeneron is not yet approved by the FDA-here's what you need to know.
According to Trump doctor, Dr. Sean Conley, in addition toPick up (An experimental drug The FDA provided approval for emergency use to treat hospitalized patients of COVID-19), zinc, vitamin D, melatonin, aspirin and famotidine (a burning medicine Estomac), the President also adopts an experimental drug not yet approved by the FDA.
According to the white house memo ", as a precautionary measure", Trump "received a dose of 8 grams of Cocktail in Regeneron Polyclonal Antibody." "He finished the infusion without incident," revealed Conley in a statement published by the White House.
Regeneron confirmed the news, adding that it had been given "a cocktail of two monoclonal antibodies" by a request for "compassionate consumption" of the president's doctors ". Read on and ensure your health and health of others, do not miss theseWithout signs that you have already had coronavirus.
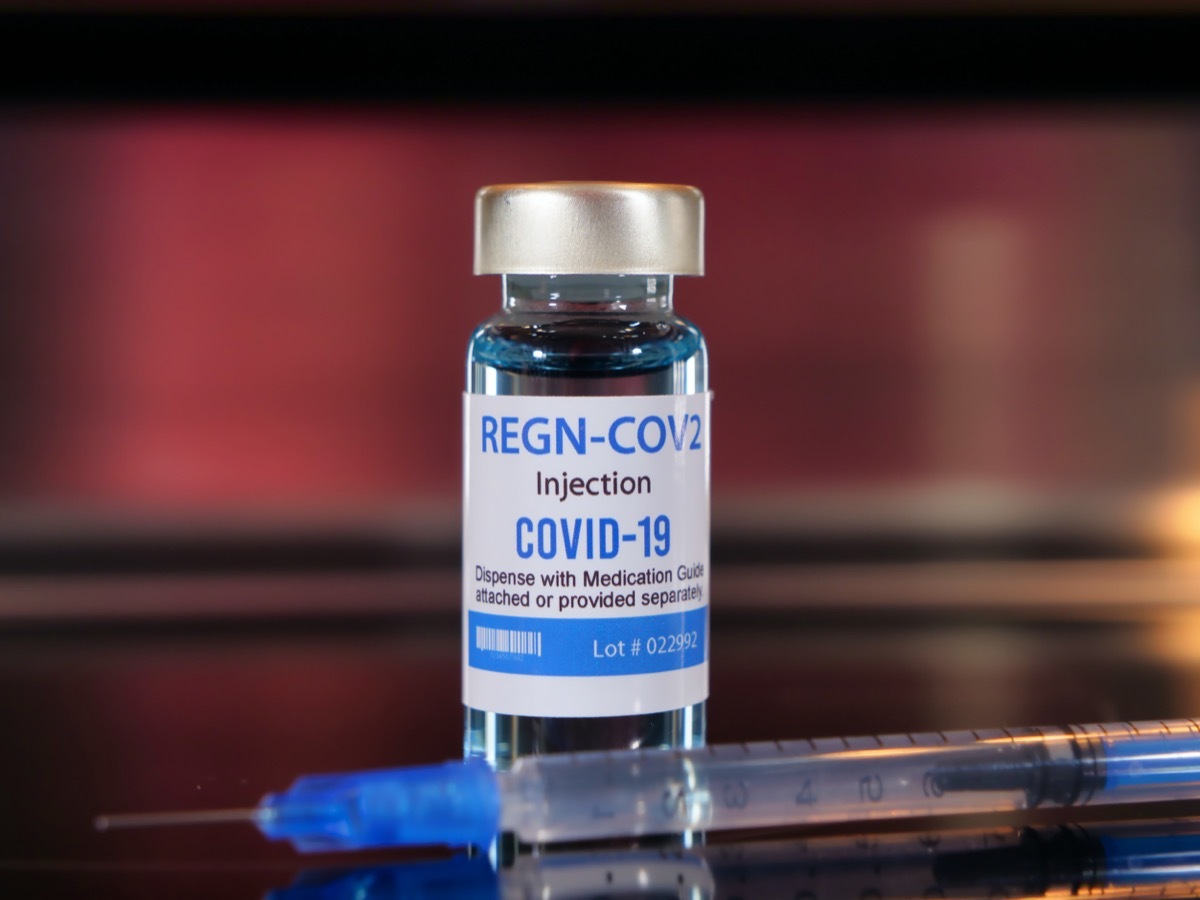
What is the regeneron?
Regeneron's treatment is officially known as REGN-COV2 and involves using monoclonal antibodies to strengthen the immune system response to the virus.
Although the polyclonal antibodies are made with several different immune cells, monoclonal antibodies involve identical immune cells that are clones of a specific mother cell.
REGN-COV2 involves a combination of two monoclonal antibodies, harvested from genetically modified mice as well as humans.
"Developing REGN-COV2, Regeneron scientists have evaluated thousands of fully human antibodies produced by the company's Velocimmune® mice, which have been genetically modified to have a human immune system, as well as identified antibodies of humans. Recovered from Covid-19, "says Regenton in a press release.
RELATED:CDC warns new COVID syndrome
What does the regeneration work?
Their "two powerful and neutralizing virus antibodies" work to "unrectively link the critical connection of the Virus Spike protein receptors", which explains the company, can effectively treat the virus even when it is mutated.
"Preclinical studies have shown that REGN-COV2 has reduced the amount of virus and damage associated with non-human primate lungs," they maintained, citingproof of their testsPublished this week, involving 275 non-hospitalized patients. The study revealed that, in addition to reducing the viral load of the virus, it was safe and improved symptoms in patients.
According to George D. Yancopoulos, MD, Ph.D., President and Head of the Regeneron Scientific Officer, the treatment is the most beneficial of "patients who had not mounted his own effective immune response, which suggests that REGN -COV2 could provide a therapeutic substitute for the natural immune response. "
"These patients were less likely to clarify the virus themselves and were more risky of prolonged symptoms." As for yourself: go through this pandemic at your healthier, do not miss these35 places you are most likely to catch Covid.

Advances Covid this state hardest hit may be inaccurate, say officials

