The FDA says "Do not get" COVID vaccine if you have this condition
Those who have "serious allergic reactions" should remain cautiously, says the FDA.
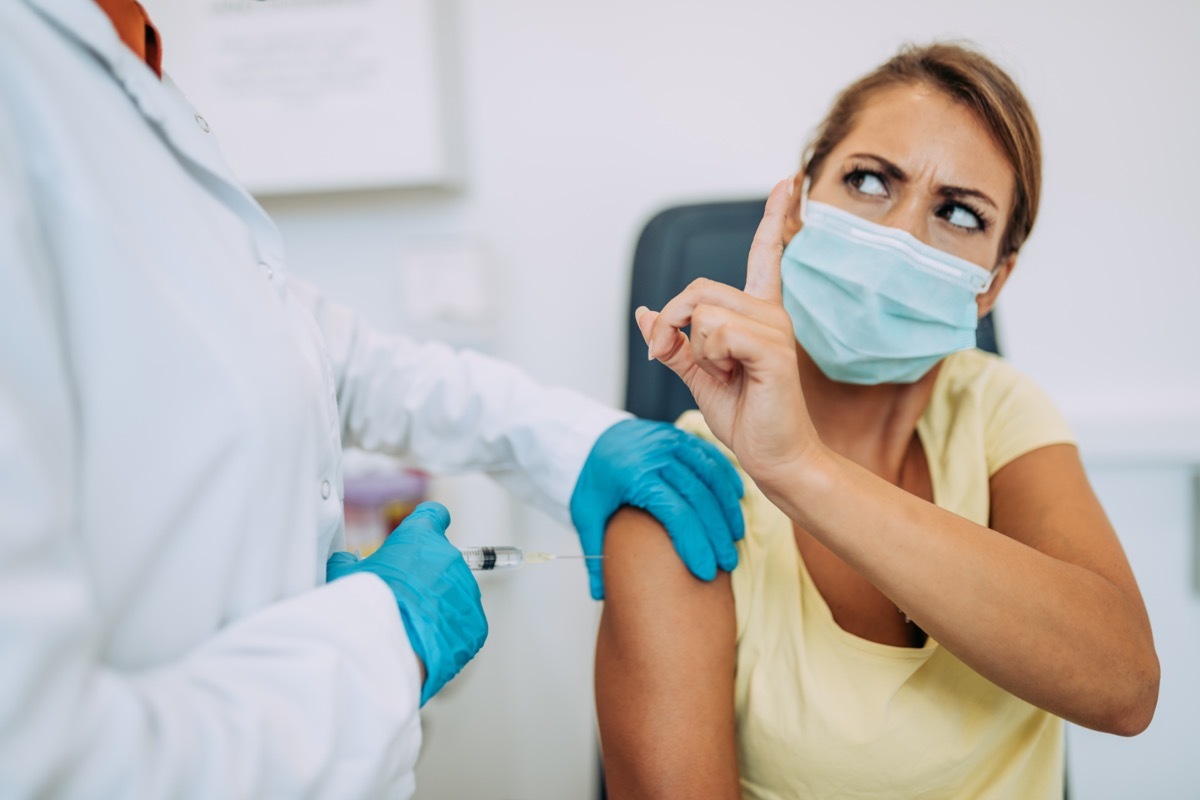
Food and drug administration (FDA) Approves the security ofvaccinesand their feelings on the vaccine against Pfizer are clear: "The FDA has evaluated and analyzed the safety and efficiency data of clinical trials carried out in tens of thousands of study participants and manufacturing information submitted by Pfizer -Biontech "and found" clear evidence that Pfizer-Biontech the Covid-19 vaccine can be effective to preventCOVID-19 [Feminine and argue that the known and potential benefits outweigh the known and potential risks of the use of the vaccine. "So what are these risks? Read to see who should see who shouldnot get the vaccine and to ensure your health and health of others, do not miss these Without signs that you have already had coronavirus.
Here who should not get the vaccine, says the FDA

You may have heard that a small number of people had serious allergic reactions to the anti-Pfizer vaccine. "Serious allergic reactions, including anaphylaxis, have been reported after the administration of the Pfizer-Biontech Covid-19 vaccine during mass vaccination outside the clinical trial frame," said the FDA. Therefore: "You should not get the Covid-19 Pfizer-Biontech Covid-19 vaccine if you:
- had a severe allergic reaction after a previous dose of this vaccine
- had a severe allergic reaction to any ingredient of this vaccine. "
"What people of Pfizer say is that if you have a history of a severe allergic reaction, you do not have to take this vaccine, or if you take it, take it in the context of a place where you develop an allergic reaction, it could be treated easily and efficiently, "saidDr. Anthony Fauciin aCNBC back healthy livestream. Keep reading to see what exactly in the vaccine, see if you could be allergic.
So what is in the vaccine?

Says the FDA: "The Pfizer-Biontech Covid-19 Covid-19 vaccine includes the following ingredients: mRNA, lipid ((4-hydroxybutyl) azanediyl) bis (hexane-6,1-diyl) bis (2-hexyldecanoate), 2 [(polyethylene glycol) -2000] -n, n-dtetroDylacetamide, 1,2-dretaroyl-sn-glyceros-3- phosphocoline and cholesterol), potassium chloride, monobasic potassium phosphate, sodium chloride, dihydrate dihydrate dihydrate sodium dihydrate And sucrose. "Then see what you need to tell your vaccine administrator before getting yours.
What you should tell your administrator of your vaccine before getting the vaccine

According to the FDA, "tells the vaccination provider about all your medical conditions, including if you:
- allergy
- have a fever
- have a bleeding disorder or are on a thinner muzzle
- are immunocomized or are on a medicine that affects your immune system
- are pregnant or plan to become pregnant
- breastfeeding
- received another Covid-19 vaccine. "
Discuss your case with your doctor, if you have any allergy problems

The CDC has good advice for those who do not know: "If you had an immediate allergic reaction, even if it did not matter to a vaccine or injectable treatment for another disease, ask your doctor if you Should get a Covid-19 vaccine. Your doctor will help you decide if you are safe for vaccinating you, "they explain. In addition, those with a polyethylene glycol allergy (PEG) or polysorbate should also avoid Get. "These recommendations include an ankle and polysorbate allergic reactions. Polysorbate is not an ingredient in the Covid-19 mRNA vaccine, but is closely linked to the ankle, which is in the vaccines. People who are allergic to duffes or polysorbate should not get a Vaccine Covid -19 arna, "they explain.
RELATED: Dr. Faisci just said when we would be returned to normal
Serious adverse events are rare

The FDA affirms "serious adverse events, while uncommon (<1.0%), were observed at slightly higher digital rates in the vaccine study group compared to the Placebo Saline Study Group, in the Together and for some specific adverse events occurring in very small numbers, "says FDA. "These represented common medical events that occur in the general population at a similar frequency. After a subsequent evaluation of the FDA, these imbalances do not constitute a security concern, they do not suggest a causal relationship to vaccination for the vast majority of serious adverse events reported.
The serious adverse effects considered by the FDA are linked to the vaccination or vaccination procedure. A case of injury to the shoulder on the vaccination site and a light lymphatic ganglion case in the armpit in front of the vaccination arm. "
Thus, except that allergies are vaccinated when they become available for you and to protect your life and the lives of others, do not visit these35 places you are most likely to catch Covid .

9 things to know before starting the Mediterranean diet

